Calculations Involving Specific Heat Worksheet
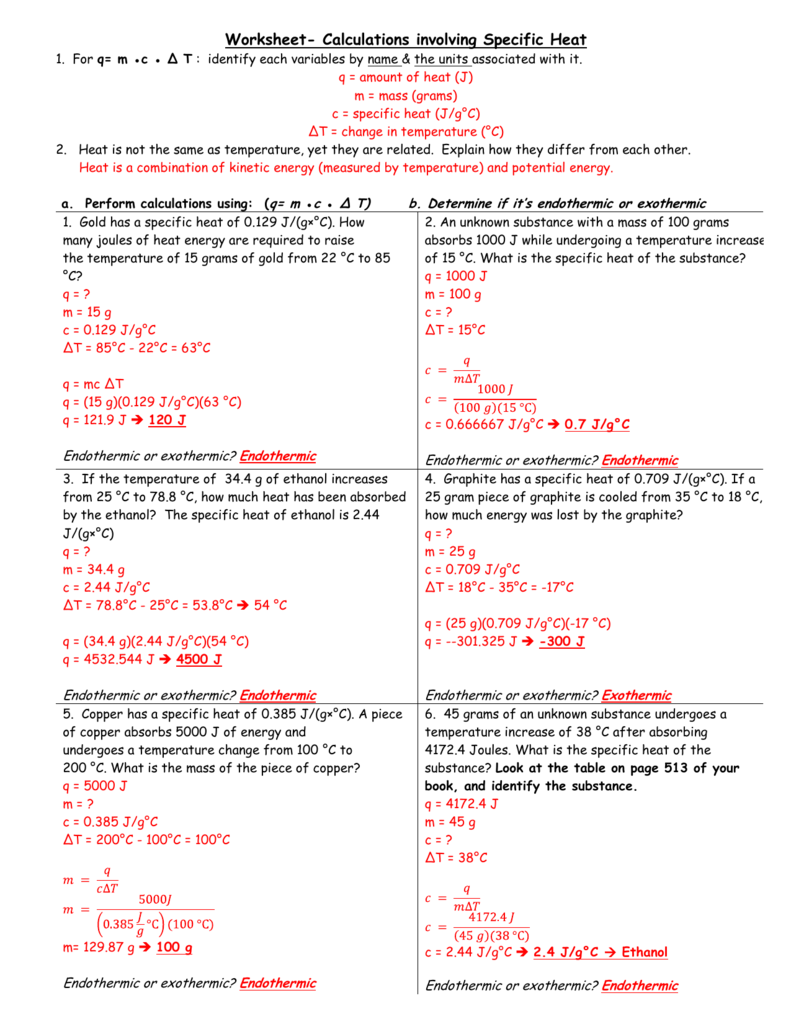
Calculations Involving Specific Heat Worksheet - For q= m c δ t : For q = m ⋅ c ⋅ δt : How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. How much heat did this. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Calculate the specific heat capacity of iron. Calculate the specific heat capacity of iron. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Identify each variable by name & the units associated with it 2. Identify each variables by name & the units associated with it.
How much heat did this. Calculate the specific heat capacity of iron. Identify each variable by name & the units associated with it 2. For q= m c δ t : How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Identify each variables by name & the units associated with it. For q = m ⋅ c ⋅ δt : Calculate the specific heat capacity of iron. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to.
How much heat did this. Identify each variables by name & the units associated with it. Calculate the specific heat capacity of iron. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. For q = m ⋅ c ⋅ δt : How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. For q= m c δ t : Identify each variable by name & the units associated with it 2. Calculate the specific heat capacity of iron.
Worksheet Calculations involving Specific Heat
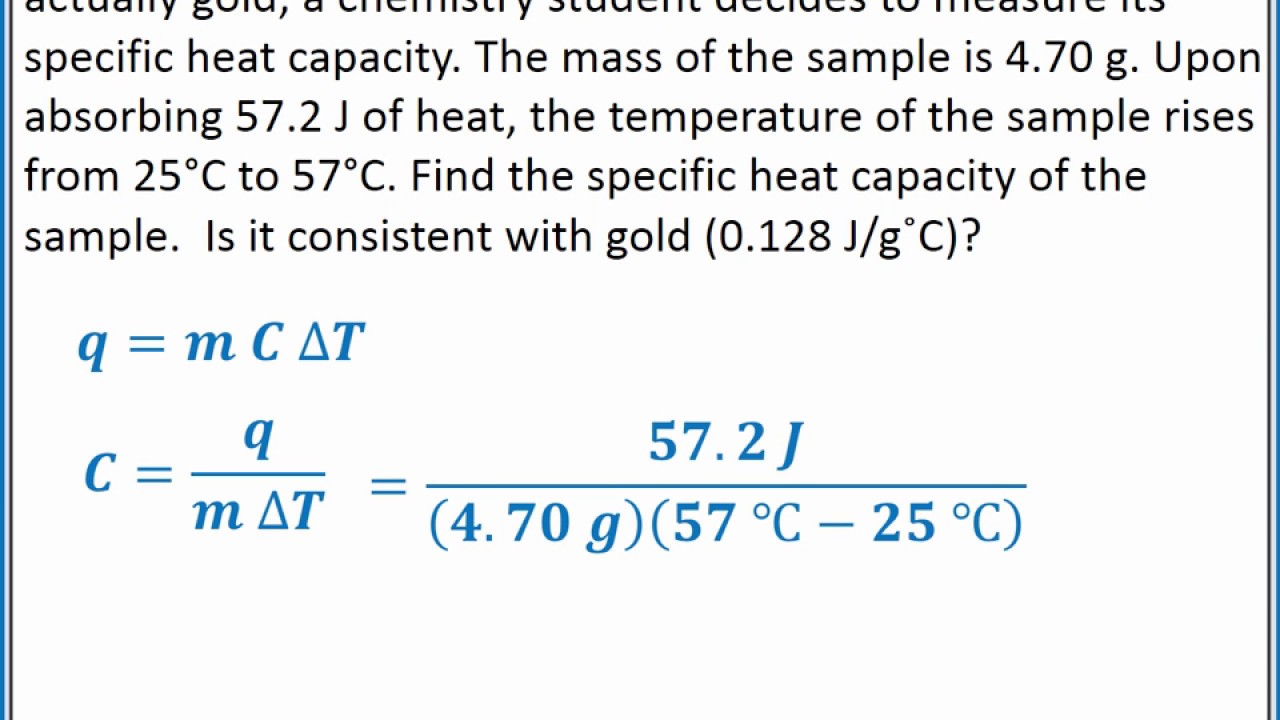
How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Calculate the specific heat capacity of iron. For q = m ⋅ c ⋅ δt : How much heat did this. For q= m c δ t :
Calculating Specific Heat Extra Practice Worksheet
Identify each variables by name & the units associated with it. For q= m c δ t : Identify each variable by name & the units associated with it 2. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. How much heat did this.
Calculations involving Specific Heat Worksheet Exercises
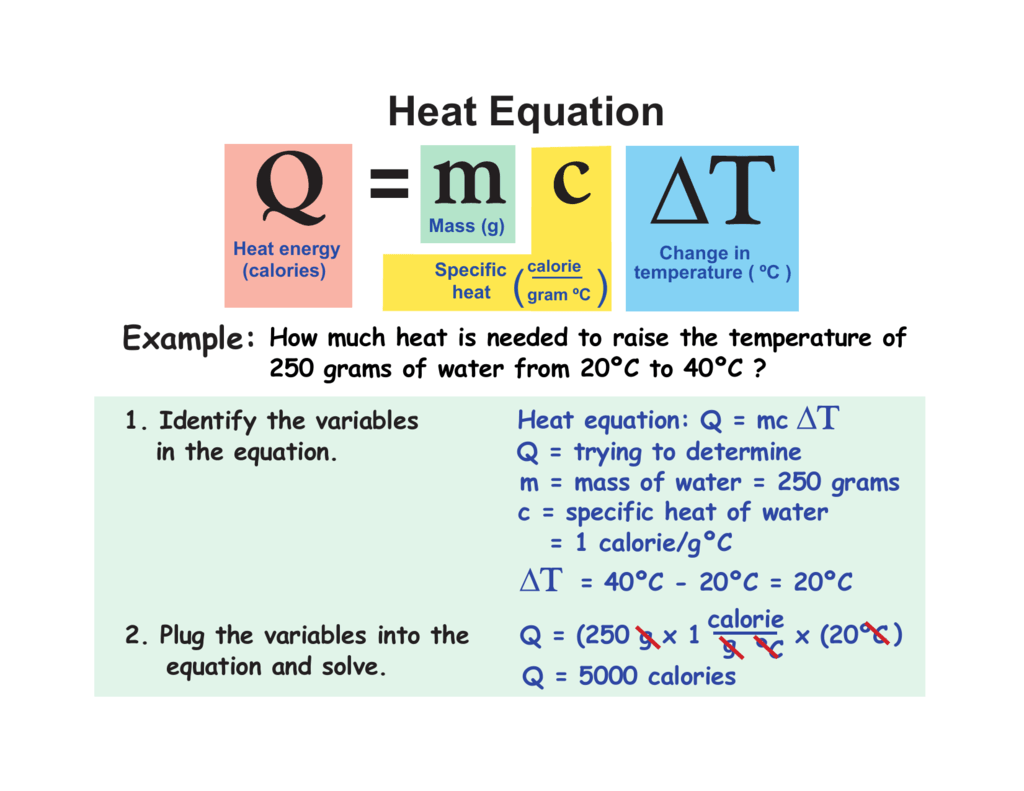
How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Calculate the specific heat capacity of iron. How much heat did this. Identify each variables by name & the units associated with it. Calculate the specific heat capacity of iron.
Worksheet Calculation Involving Specific Heat
How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. How much heat.
Calculating Specific Heat Worksheet
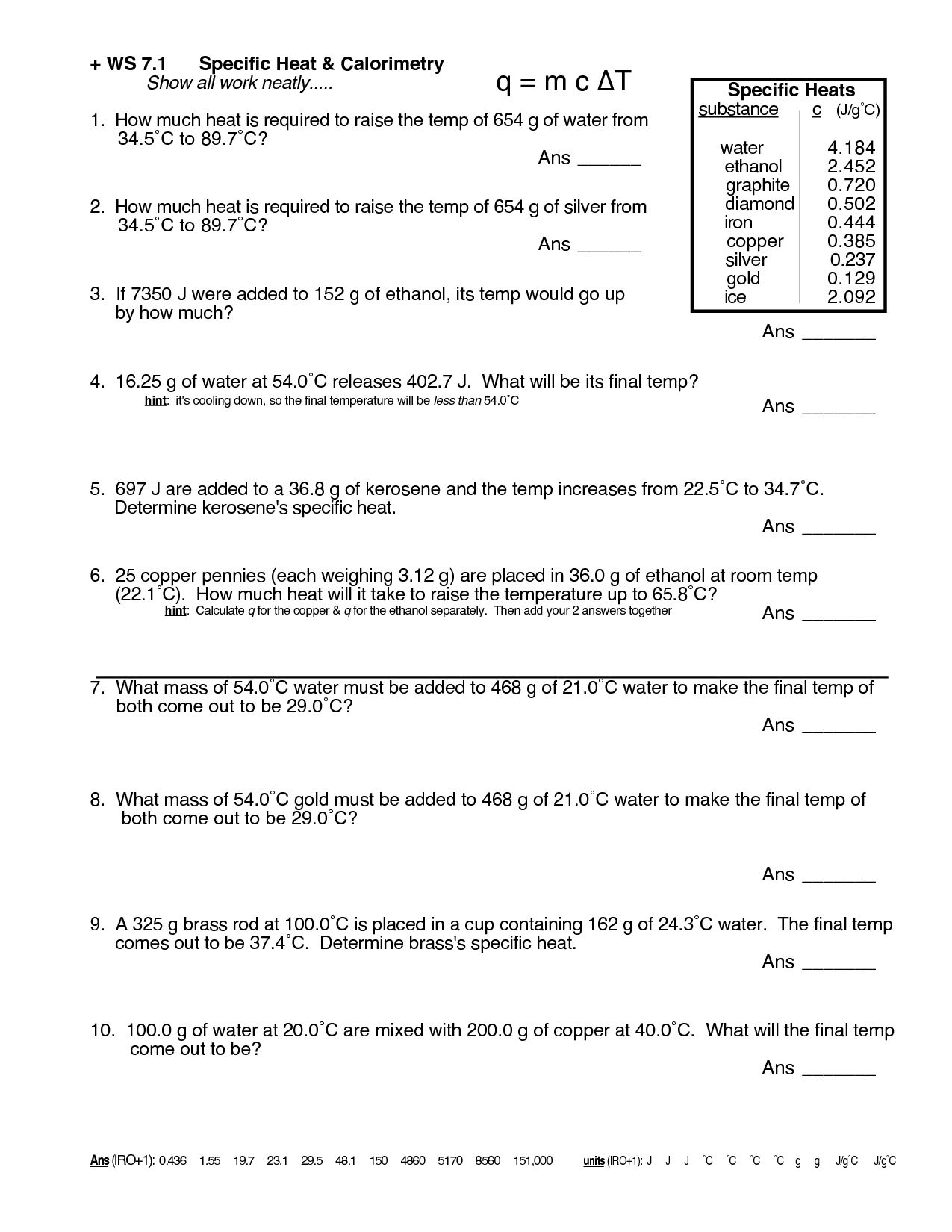
For q= m c δ t : How much heat did this. Identify each variables by name & the units associated with it. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. For q = m ⋅ c ⋅ δt :
Specific Heat Calculations Interactive Worksheet by Cheryl Thomas
Calculate the specific heat capacity of iron. For q= m c δ t : Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Identify each variables by name & the units associated with it. Identify each variable by name & the units associated with it 2.
10++ Specific Heat Worksheet Worksheets Decoomo
For q= m c δ t : Identify each variables by name & the units associated with it. Identify each variable by name & the units associated with it 2. How much heat did this. Calculate the specific heat capacity of iron.
Specific heat calculationsconverted Part 1 Calculating
Calculate the specific heat capacity of iron. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. For q= m c δ t : Identify each variable by name & the units.
Worksheet Calculations Involving Specific Heat
Identify each variable by name & the units associated with it 2. Calculate the specific heat capacity of iron. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. How much heat did this. Calculate the specific heat capacity of iron.
Worksheet Calculation Involving Specific Heat
Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. How much heat did this. For q= m c δ t : How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Calculate the specific heat capacity of iron.
Identify Each Variable By Name & The Units Associated With It 2.
Identify each variables by name & the units associated with it. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Calculate the specific heat capacity of iron. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc.
For Q = M ⋅ C ⋅ Δt :
How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. How much heat did this. For q= m c δ t : Calculate the specific heat capacity of iron.