Clinical Study Protocol Template
Clinical Study Protocol Template - Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol. The natural history/observational protocol template, the. There are three templates to be used for observational research: Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:.
There are three templates to be used for observational research: The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The natural history/observational protocol template, the. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and.
Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol. There are three templates to be used for observational research: Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. The natural history/observational protocol template, the.
Clinical Protocol Template Master of Documents
Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. There are three templates to be used.
Clinical Trial Protocol Synopsis Template
The natural history/observational protocol template, the. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. The study designs page includes numerous basic journal articles linked to.
Trial Report Template
There are three templates to be used for observational research: The natural history/observational protocol template, the. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. Nih applicants can use a template with instructional and sample.
Clinical Protocol Template Master of Documents
The natural history/observational protocol template, the. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. There are three templates to be used for observational research: Nih applicants can use a.
Clinical Study Protocol Template
The natural history/observational protocol template, the. There are three templates to be used for observational research: The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. Nih applicants can use a template with instructional and sample text to help.
Clinical Study Protocol PowerPoint And Google Slides, 53 OFF
The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol. There are three templates to be used for observational research: Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The natural history/observational protocol template, the. The study designs page includes numerous basic journal articles.
Clinical Study Protocol PowerPoint and Google Slides Template PPT Slides
The natural history/observational protocol template, the. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. There are three templates to be used for observational research: Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. The.
Study Protocol Template
Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol. The natural history/observational protocol template, the. There.
Clinical Study Protocol (CSP) Template Clinical Study Templates
The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. The natural history/observational protocol template, the. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. Nih applicants can use a template with instructional and sample text to help write clinical.
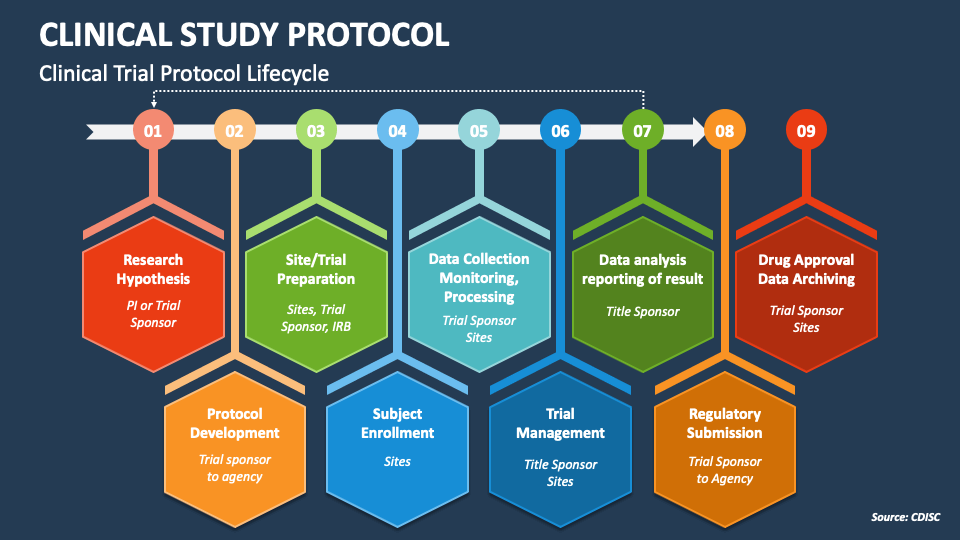
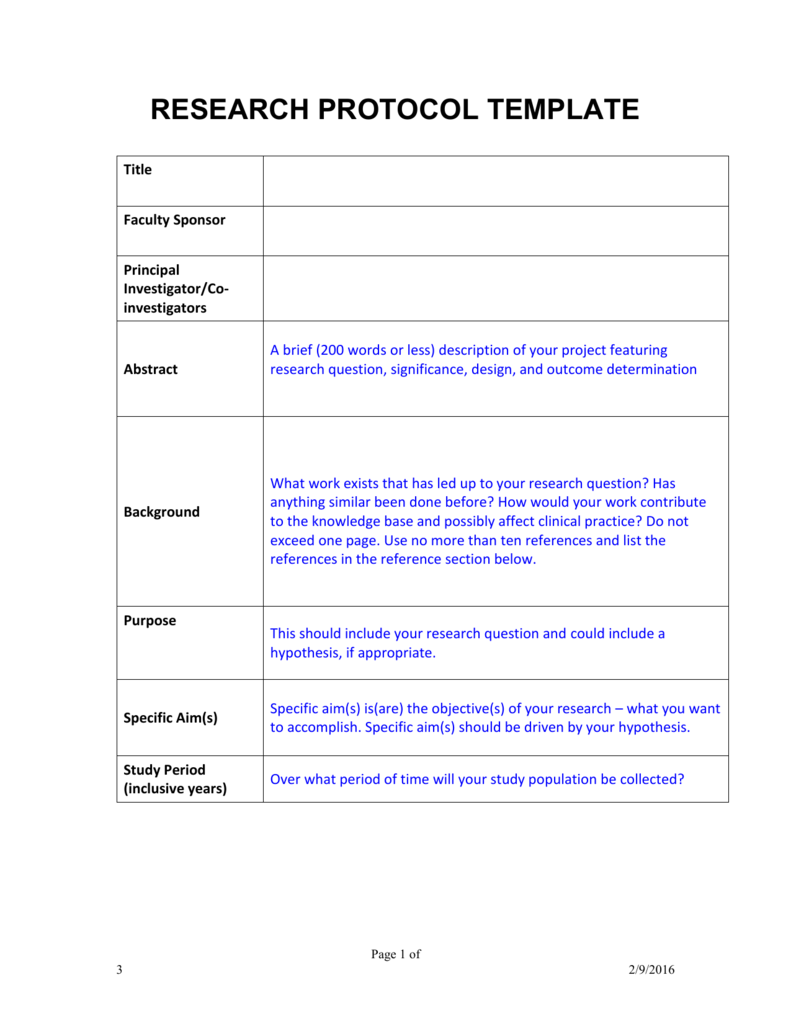
research protocol template
The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. The ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol. There are three templates to be used for.
The Ich M11 Clinical Electronic Structured Harmonised Protocol Template Provides Comprehensive Clinical Protocol.
The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. The natural history/observational protocol template, the. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. There are three templates to be used for observational research: