Content Of Human Factors Information In Medical Device Marketing Submissions
Content Of Human Factors Information In Medical Device Marketing Submissions - Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
New FDA Guidance for Medical Device Human Factors Research Collective
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
10 Essential Human Factors in Medical Device Marketing Submissions 2023
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
FDA'S Human Factors Premarket Evaluation guidance
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
Medical Device Research
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
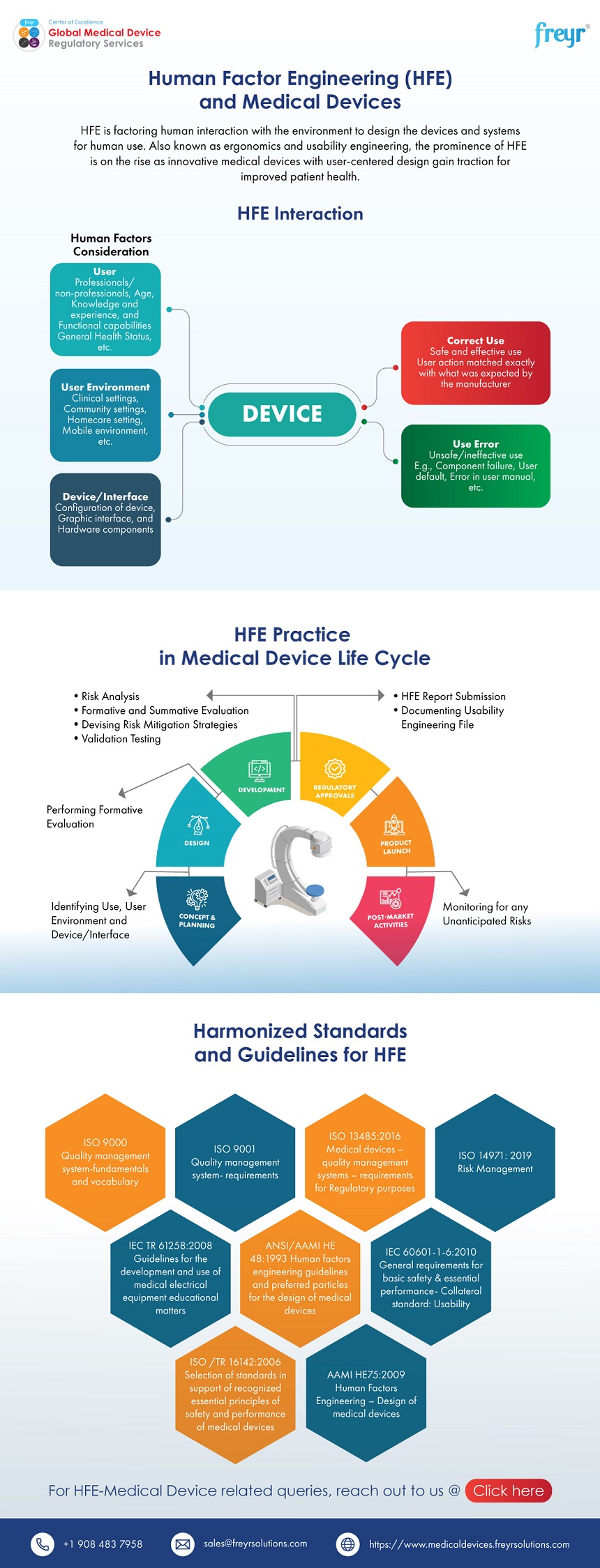
Human Factor Engineering (HFE) and Medical Devices Freyr Global
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
FDA Clarifies the Human Factors or Usability Information to Include in
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
FDA Human Factors Guidance Draft What Medical Device Manufacturers
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
FDA Human Factors Guidance Framework for Human Factors Information in
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.
Understanding FDA’s New Guidance Document on Human Factors Information
Center for devices and radiological health (cdrh) draft guidance content of human factors information in medical device marketing.