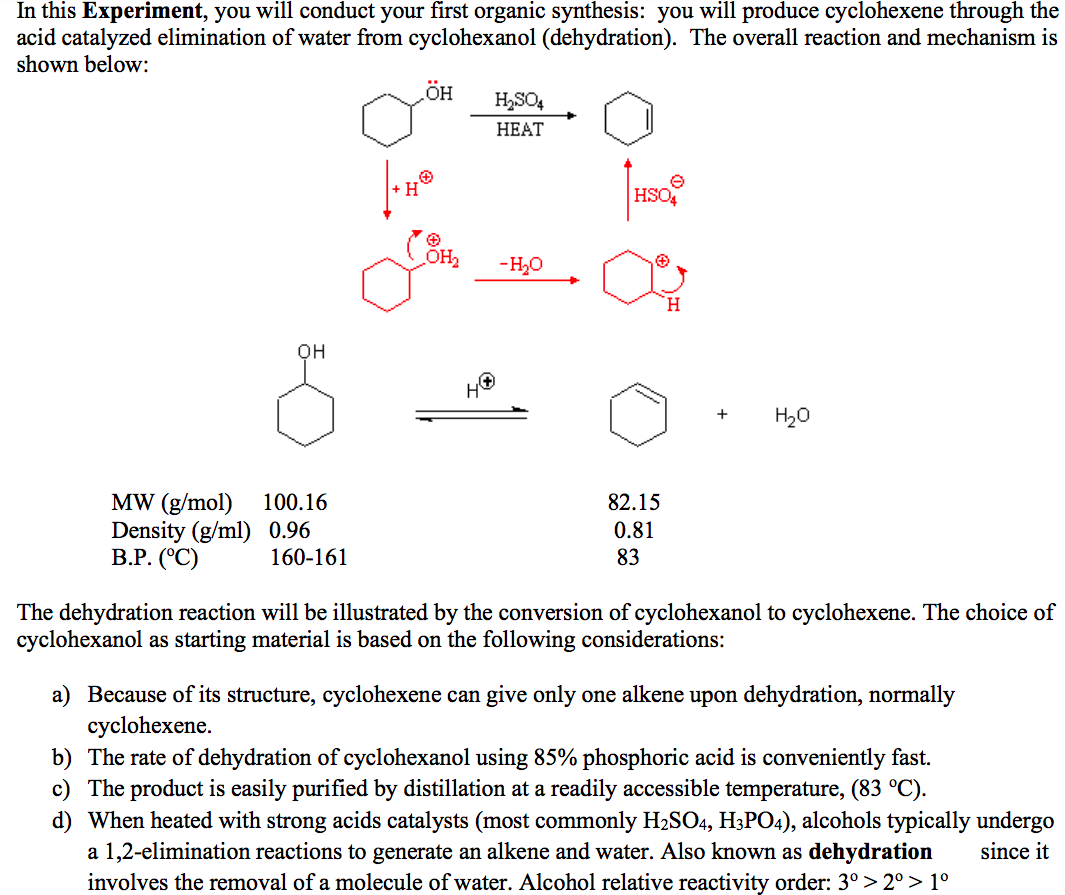
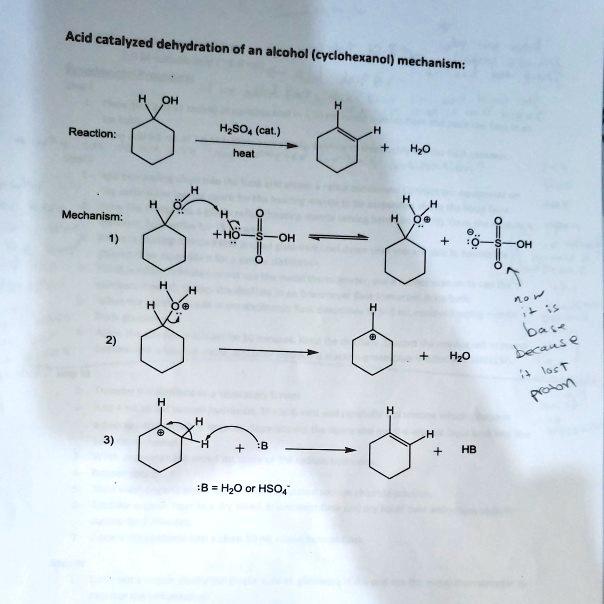
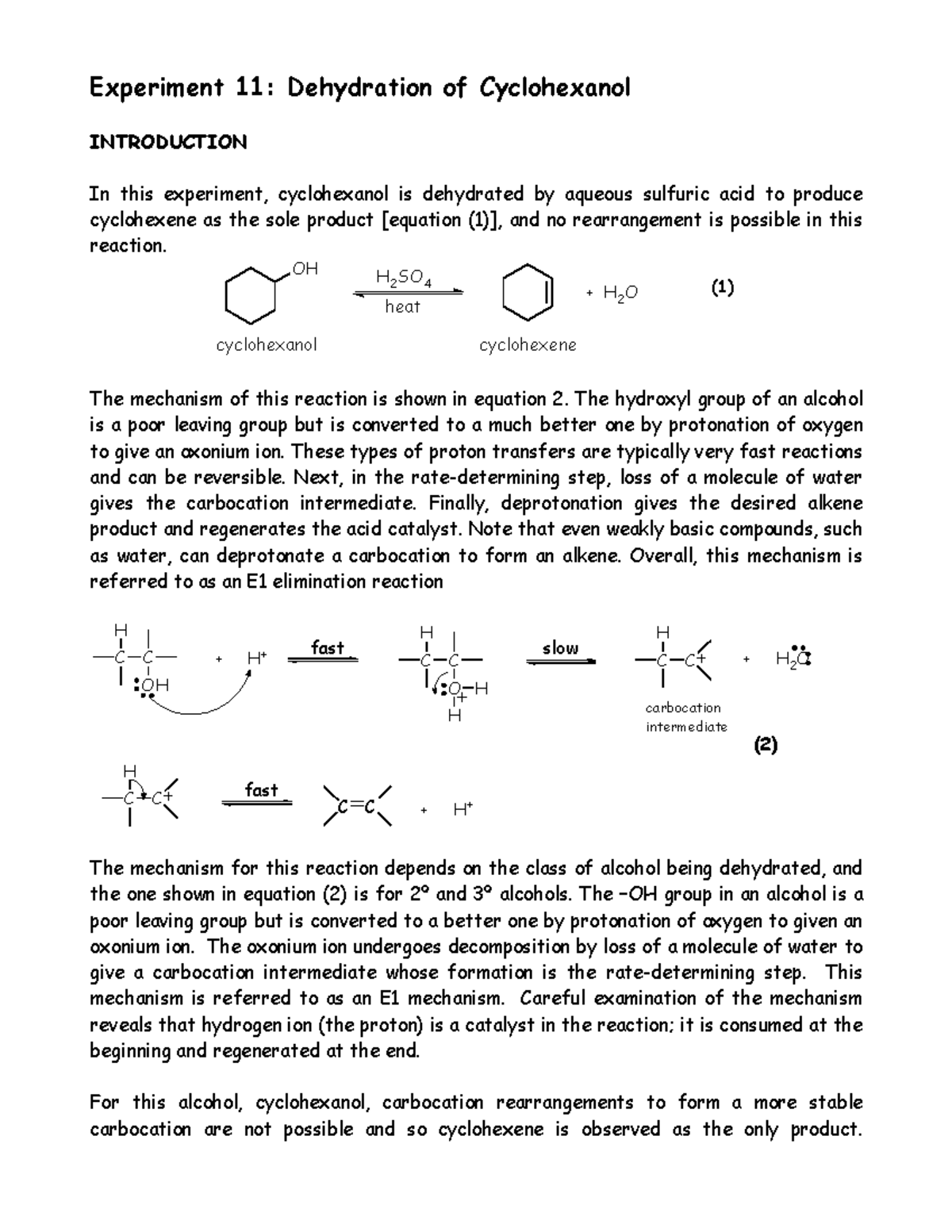
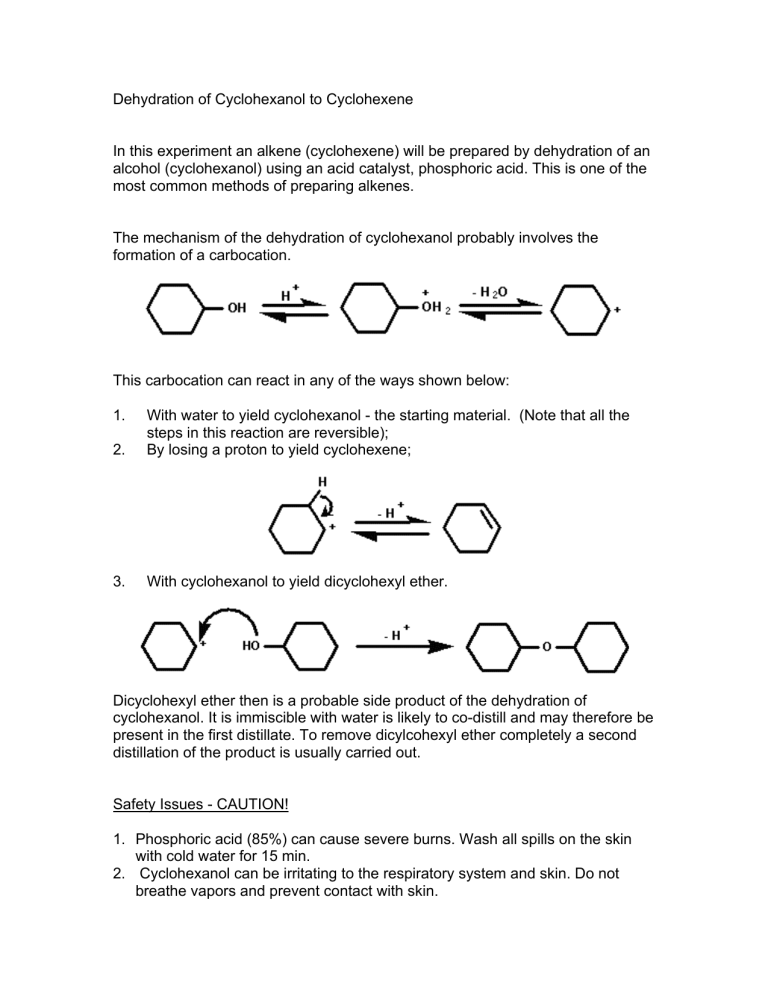
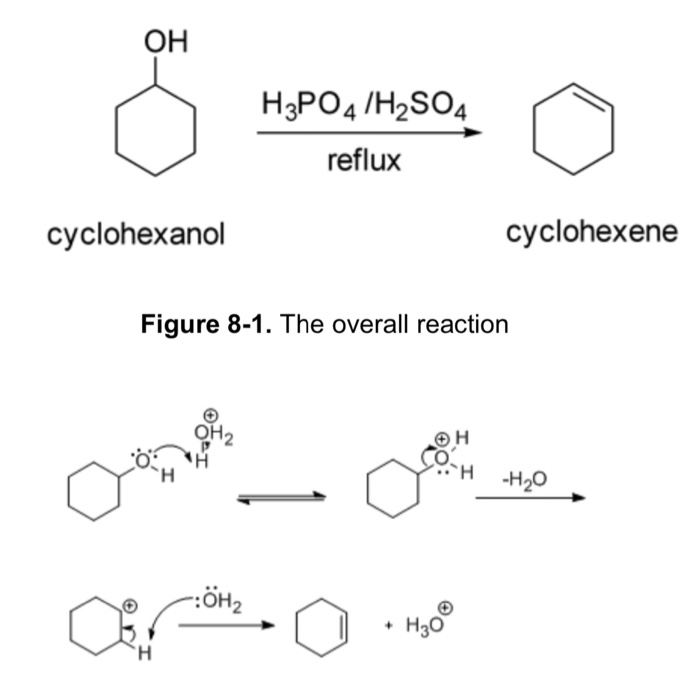
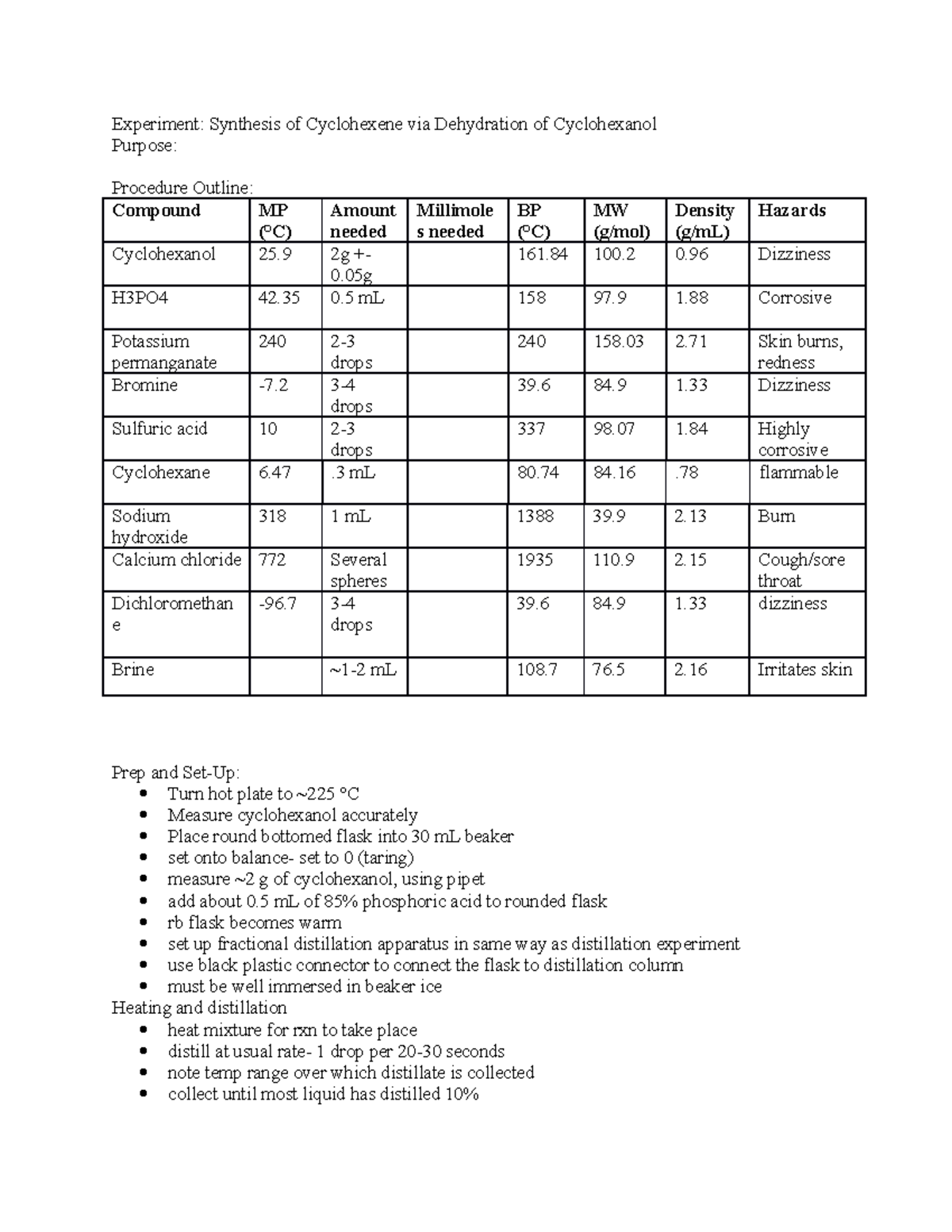
Dehydration Of Cyclohexanol To Cyclohexene
Dehydration Of Cyclohexanol To Cyclohexene - The cyclohexene product will be isolated by simple. Theoretically, in a dehydration reaction, one mole of alcohol. This is a preparation commonly used to illustrate the formation and. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. The dehydration of cyclohexanol to yield cyclohexene.
Theoretically, in a dehydration reaction, one mole of alcohol. The dehydration of cyclohexanol to yield cyclohexene. The cyclohexene product will be isolated by simple. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. This is a preparation commonly used to illustrate the formation and.
From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. Theoretically, in a dehydration reaction, one mole of alcohol. The cyclohexene product will be isolated by simple. The dehydration of cyclohexanol to yield cyclohexene. This is a preparation commonly used to illustrate the formation and.
Cyclohexanol Dehydration
The dehydration of cyclohexanol to yield cyclohexene. Theoretically, in a dehydration reaction, one mole of alcohol. This is a preparation commonly used to illustrate the formation and. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. The cyclohexene product will be isolated by simple.
Cyclohexanol Dehydration
The dehydration of cyclohexanol to yield cyclohexene. Theoretically, in a dehydration reaction, one mole of alcohol. This is a preparation commonly used to illustrate the formation and. The cyclohexene product will be isolated by simple. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced.
dehydration of cyclohexane Experiment 11 Dehydration of Cyclohexanol
This is a preparation commonly used to illustrate the formation and. Theoretically, in a dehydration reaction, one mole of alcohol. The cyclohexene product will be isolated by simple. The dehydration of cyclohexanol to yield cyclohexene. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced.
Synthesis of Cyclohexene via Dehydration of Cyclohexanol lab
This is a preparation commonly used to illustrate the formation and. The cyclohexene product will be isolated by simple. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. Theoretically, in a dehydration reaction, one mole of alcohol. The dehydration of cyclohexanol to yield cyclohexene.
[Solved] Synthesis of Cyclohexene by Dehydration of Cyclohexanol There
This is a preparation commonly used to illustrate the formation and. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. The cyclohexene product will be isolated by simple. Theoretically, in a dehydration reaction, one mole of alcohol. The dehydration of cyclohexanol to yield cyclohexene.
Cyclohexene from Cyclohexanol Dehydration of alcohol YouTube
Theoretically, in a dehydration reaction, one mole of alcohol. The dehydration of cyclohexanol to yield cyclohexene. This is a preparation commonly used to illustrate the formation and. The cyclohexene product will be isolated by simple. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced.
Practical skills assessment video the dehydration of cyclohexanol to
The cyclohexene product will be isolated by simple. Theoretically, in a dehydration reaction, one mole of alcohol. This is a preparation commonly used to illustrate the formation and. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. The dehydration of cyclohexanol to yield cyclohexene.
Cyclohexanol Dehydration
The cyclohexene product will be isolated by simple. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. This is a preparation commonly used to illustrate the formation and. Theoretically, in a dehydration reaction, one mole of alcohol. The dehydration of cyclohexanol to yield cyclohexene.
Solved (iv). What type of reaction is the conversion of
From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. The cyclohexene product will be isolated by simple. Theoretically, in a dehydration reaction, one mole of alcohol. The dehydration of cyclohexanol to yield cyclohexene. This is a preparation commonly used to illustrate the formation and.
Pre lab 6 Synthesis of Cyclohexene via Dehydration of Cyclohexanol
The cyclohexene product will be isolated by simple. The dehydration of cyclohexanol to yield cyclohexene. Theoretically, in a dehydration reaction, one mole of alcohol. This is a preparation commonly used to illustrate the formation and. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced.
The Dehydration Of Cyclohexanol To Yield Cyclohexene.
Theoretically, in a dehydration reaction, one mole of alcohol. From a 6 ml of cyclohexanol, 2 grams of cyclohexene is produced. This is a preparation commonly used to illustrate the formation and. The cyclohexene product will be isolated by simple.