How Many Bonds Does Chlorine Form
How Many Bonds Does Chlorine Form - Each chlorine can form 1 bond. Each magnesium can form 2 bonds. This is because chlorine is a highly electronegative element, which means it. Chlorine can form a maximum of one covalent bond with other elements. Each sodium can form 1 bond. Chlorine typically forms one bond with other elements. Chlorine can form three main types of bonds: However, it can also form ionic bonds in certain. Each calcium can form 2 bonds. Chlorine forms single bonds with elements such as carbon, oxygen,.
Each chlorine can form 1 bond. Chlorine can form three main types of bonds: Chlorine typically forms one bond with other elements. The bonds formed between chlorine atoms of molecule will be covalent bond and bonded by one covalent bond only. Each calcium can form 2 bonds. Chlorine can form a maximum of one covalent bond with other elements. Chlorine forms single bonds with elements such as carbon, oxygen,. Each sodium can form 1 bond. Each magnesium can form 2 bonds. However, it can also form ionic bonds in certain.
Chlorine can form a maximum of one covalent bond with other elements. Each sodium can form 1 bond. Each calcium can form 2 bonds. This is because chlorine is a highly electronegative element, which means it. Chlorine typically forms one bond with other elements. Chlorine can form three main types of bonds: Chlorine forms single bonds with elements such as carbon, oxygen,. Each chlorine can form 1 bond. The bonds formed between chlorine atoms of molecule will be covalent bond and bonded by one covalent bond only. However, it can also form ionic bonds in certain.
Which diagram shows how the covalent bonds most likely form in a
Each calcium can form 2 bonds. Chlorine can form a maximum of one covalent bond with other elements. This is because chlorine is a highly electronegative element, which means it. Each sodium can form 1 bond. Chlorine typically forms one bond with other elements.
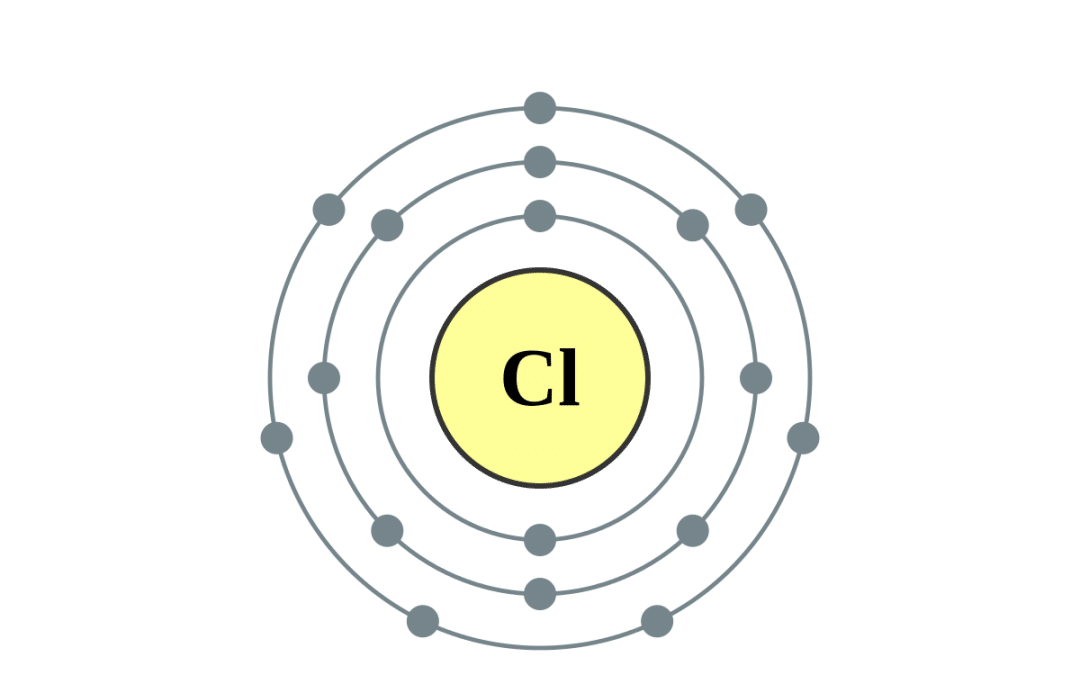
Chlorine Diagram
Each sodium can form 1 bond. Chlorine can form a maximum of one covalent bond with other elements. Chlorine typically forms one bond with other elements. Chlorine can form three main types of bonds: This is because chlorine is a highly electronegative element, which means it.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Each calcium can form 2 bonds. Each chlorine can form 1 bond. Each magnesium can form 2 bonds. This is because chlorine is a highly electronegative element, which means it. Chlorine can form three main types of bonds:
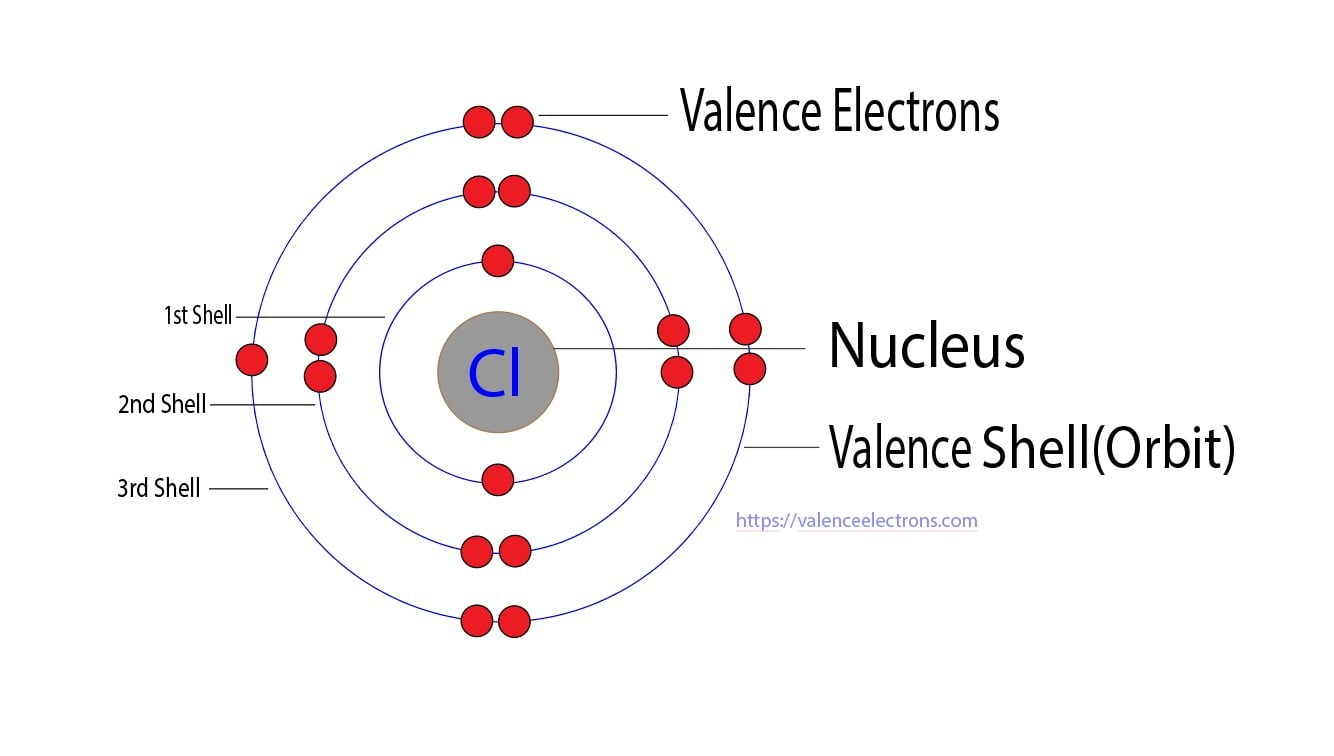
How Many Valence Electrons Does Chlorine (Cl) Have?
Chlorine can form three main types of bonds: The bonds formed between chlorine atoms of molecule will be covalent bond and bonded by one covalent bond only. Chlorine typically forms one bond with other elements. Each magnesium can form 2 bonds. Each calcium can form 2 bonds.
SOLVED What are isomers? Draw the isomers of 2butanol. How many
Chlorine forms single bonds with elements such as carbon, oxygen,. Each magnesium can form 2 bonds. Chlorine typically forms one bond with other elements. Each calcium can form 2 bonds. Each chlorine can form 1 bond.
SOLVED 2) How many bonds does a molecule of chlorine form? (2 pts) 3
Chlorine forms single bonds with elements such as carbon, oxygen,. Chlorine can form three main types of bonds: Each magnesium can form 2 bonds. Each calcium can form 2 bonds. Chlorine typically forms one bond with other elements.
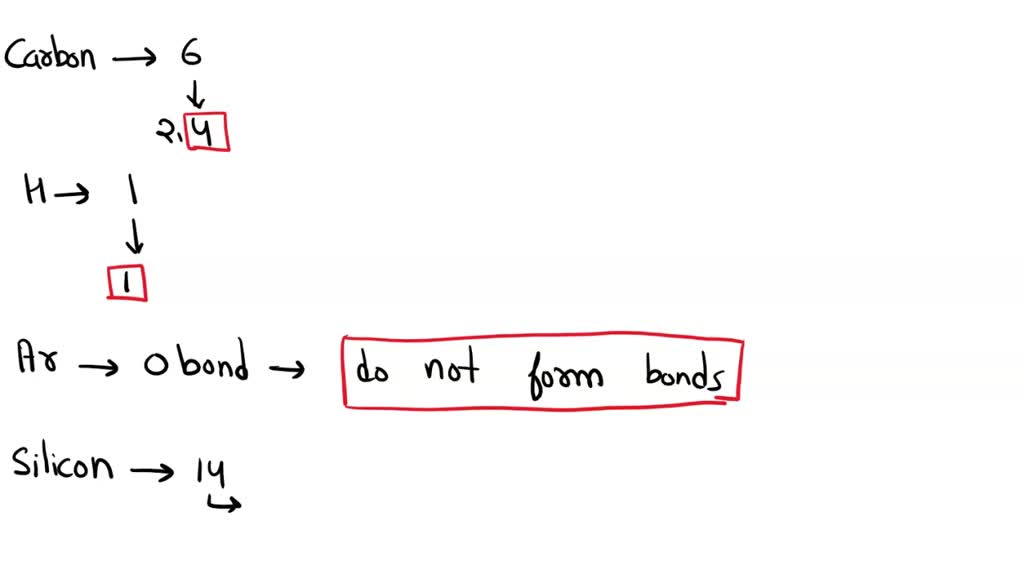
How to predict number of bonds each element makes Predictions
Each magnesium can form 2 bonds. Chlorine can form a maximum of one covalent bond with other elements. Each chlorine can form 1 bond. Each calcium can form 2 bonds. The bonds formed between chlorine atoms of molecule will be covalent bond and bonded by one covalent bond only.
15 Interesting Facts About Chlorine
Chlorine can form three main types of bonds: The bonds formed between chlorine atoms of molecule will be covalent bond and bonded by one covalent bond only. Each chlorine can form 1 bond. This is because chlorine is a highly electronegative element, which means it. Chlorine can form a maximum of one covalent bond with other elements.
Cl2 Molecular Geometry
The bonds formed between chlorine atoms of molecule will be covalent bond and bonded by one covalent bond only. Each calcium can form 2 bonds. Each magnesium can form 2 bonds. Chlorine forms single bonds with elements such as carbon, oxygen,. Chlorine can form three main types of bonds:
This Is Because Chlorine Is A Highly Electronegative Element, Which Means It.
Each sodium can form 1 bond. Chlorine can form three main types of bonds: Each calcium can form 2 bonds. The bonds formed between chlorine atoms of molecule will be covalent bond and bonded by one covalent bond only.
Chlorine Typically Forms One Bond With Other Elements.
Chlorine forms single bonds with elements such as carbon, oxygen,. Each magnesium can form 2 bonds. Chlorine can form a maximum of one covalent bond with other elements. However, it can also form ionic bonds in certain.