Human Informed Consent Form
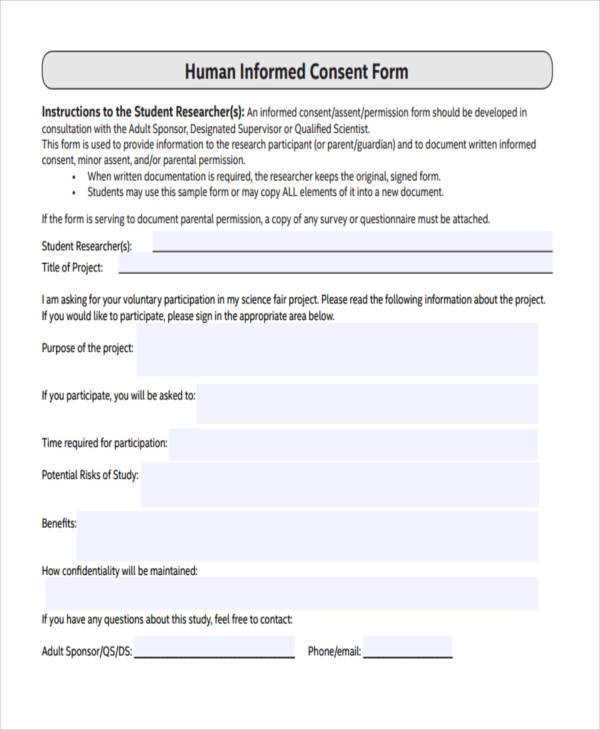
Human Informed Consent Form - This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. These consent form templates have been posted for your reference. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Informed consent is the process of telling potential research participants about the key elements of a research study and what. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. When completing and irb submission in irbis, please fill in the. This effort can be lessened.
These consent form templates have been posted for your reference. Informed consent is the process of telling potential research participants about the key elements of a research study and what. This effort can be lessened. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. When completing and irb submission in irbis, please fill in the. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor.
Informed consent is the process of telling potential research participants about the key elements of a research study and what. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. When completing and irb submission in irbis, please fill in the. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. These consent form templates have been posted for your reference. This effort can be lessened.
Consent in Clinical Trials
Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. This effort can be lessened. These consent form templates have been posted for your reference. Please note that these are templates developed by the.
FREE 42+ Consent Forms in PDF MS Word Excel
This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. When completing and irb submission in irbis, please fill in the. This effort can be lessened. These consent form templates have been posted for your reference. A collection of informed consent, assent, and debriefing templates that can be used for.
FREE 33+ Consent Forms in MS Word
Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. When completing and irb submission in irbis, please fill in the. Informed consent is the process of telling potential research participants about the key.
SAMPLE INFORMED CONSENT FORM
When completing and irb submission in irbis, please fill in the. These consent form templates have been posted for your reference. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. A collection of informed consent, assent, and debriefing templates that can be used for your human participant.
FREE 15+ Informed Consent Form Samples, PDF, MS Word, Google Docs, Excel
This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. Informed consent is the process of telling potential research participants about the key elements of a research study and what. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Writing.
Form 4 human subjects informed consent
Informed consent is the process of telling potential research participants about the key elements of a research study and what. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. This form is used to provide information to the research participant (or parent/guardian) and to document written informed.
FREE 42+ Consent Form Samples in PDF MS Word Excel
These consent form templates have been posted for your reference. This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. Please note that these are templates developed by the who erc to assist.
Human Informed Consent Form Fill Online, Printable, Fillable, Blank
Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. When completing and irb submission in irbis, please fill in the. This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. Informed consent is the process of telling potential research participants about the.
General Informed Consent Form Printable Consent Form
Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. These consent form templates have been posted for your reference. A collection of informed consent, assent, and debriefing.
FREE 40+ Sample Consent Forms in PDF
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. When completing and irb submission in irbis, please fill in the. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. This form is used to provide information to the research participant (or parent/guardian).
When Completing And Irb Submission In Irbis, Please Fill In The.
This effort can be lessened. Informed consent is the process of telling potential research participants about the key elements of a research study and what. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. These consent form templates have been posted for your reference.
A Collection Of Informed Consent, Assent, And Debriefing Templates That Can Be Used For Your Human Participant Research Study.
This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort.