Identify The Name Of The Enthalpy Change For Each Reaction
Identify The Name Of The Enthalpy Change For Each Reaction - Hess’ law is based on the addition of reactions. To fairly compare the changes in enthalpy between reactions, all reactions should be carried out. Write the overall reaction, and identify the name of the enthalpy change for each reaction. Reaction enthalpy answer bank m(s) m(g) step 1: Identify the name of the enthalpy change for each reaction. By knowing the reaction enthalpy for constituent reactions, the enthalpy of a reaction that can be.
Write the overall reaction, and identify the name of the enthalpy change for each reaction. Reaction enthalpy answer bank m(s) m(g) step 1: By knowing the reaction enthalpy for constituent reactions, the enthalpy of a reaction that can be. Hess’ law is based on the addition of reactions. Identify the name of the enthalpy change for each reaction. To fairly compare the changes in enthalpy between reactions, all reactions should be carried out.
By knowing the reaction enthalpy for constituent reactions, the enthalpy of a reaction that can be. Identify the name of the enthalpy change for each reaction. Reaction enthalpy answer bank m(s) m(g) step 1: To fairly compare the changes in enthalpy between reactions, all reactions should be carried out. Hess’ law is based on the addition of reactions. Write the overall reaction, and identify the name of the enthalpy change for each reaction.
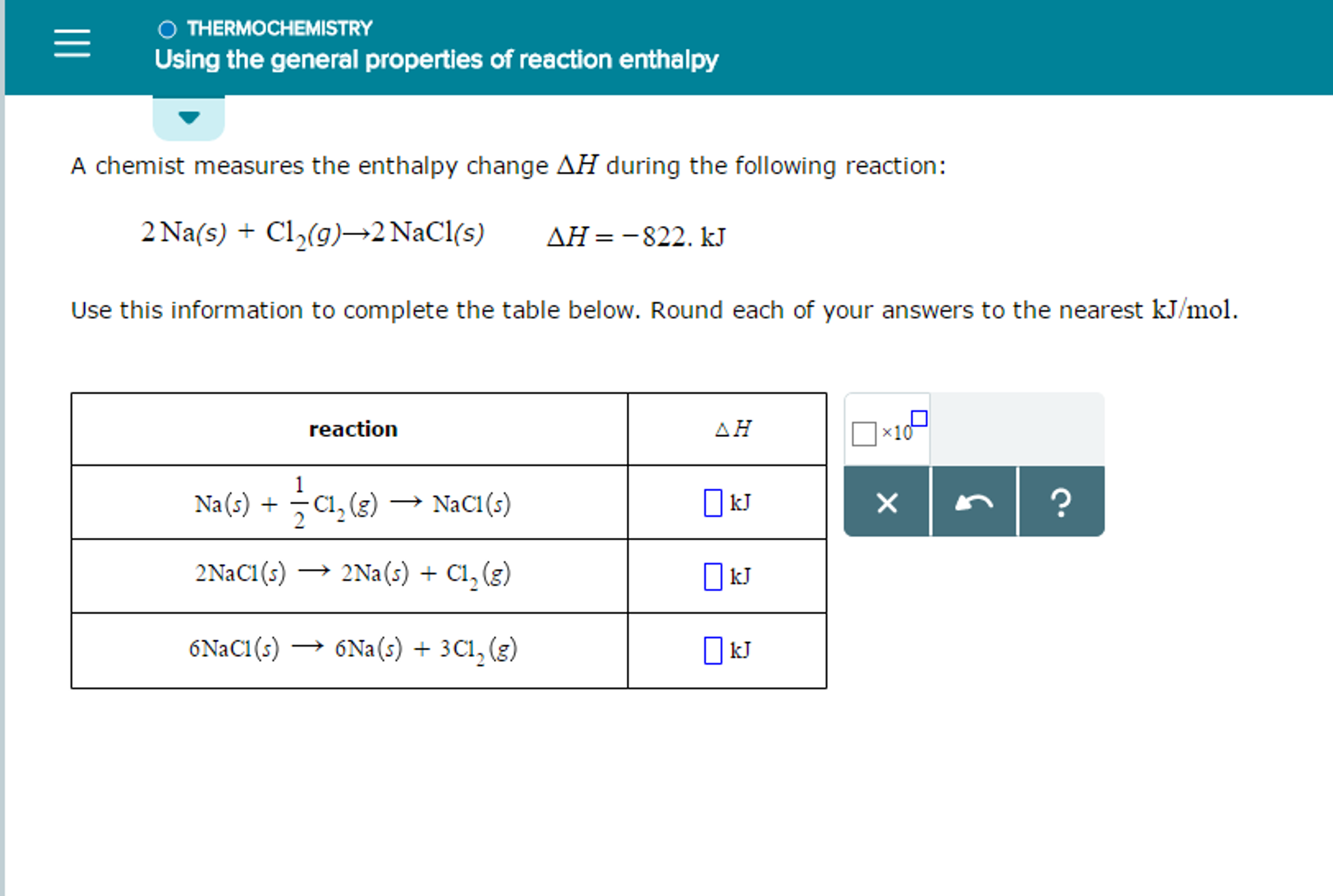
Solved A chemist measures the enthalpy change AH during the
Identify the name of the enthalpy change for each reaction. To fairly compare the changes in enthalpy between reactions, all reactions should be carried out. Write the overall reaction, and identify the name of the enthalpy change for each reaction. By knowing the reaction enthalpy for constituent reactions, the enthalpy of a reaction that can be. Hess’ law is based.
Enthalpy Introduction, Calculation, Enthalpy change, Importance
To fairly compare the changes in enthalpy between reactions, all reactions should be carried out. Identify the name of the enthalpy change for each reaction. Reaction enthalpy answer bank m(s) m(g) step 1: By knowing the reaction enthalpy for constituent reactions, the enthalpy of a reaction that can be. Hess’ law is based on the addition of reactions.
How To Calculate Enthalpy Change A Comprehensive Guide IHSANPEDIA
By knowing the reaction enthalpy for constituent reactions, the enthalpy of a reaction that can be. Reaction enthalpy answer bank m(s) m(g) step 1: Hess’ law is based on the addition of reactions. Write the overall reaction, and identify the name of the enthalpy change for each reaction. To fairly compare the changes in enthalpy between reactions, all reactions should.
How To Find Enthalpy Change Of Reaction First write the equation of
Reaction enthalpy answer bank m(s) m(g) step 1: To fairly compare the changes in enthalpy between reactions, all reactions should be carried out. Write the overall reaction, and identify the name of the enthalpy change for each reaction. Hess’ law is based on the addition of reactions. Identify the name of the enthalpy change for each reaction.
Enthalpy Change Of Formation Equation
To fairly compare the changes in enthalpy between reactions, all reactions should be carried out. By knowing the reaction enthalpy for constituent reactions, the enthalpy of a reaction that can be. Write the overall reaction, and identify the name of the enthalpy change for each reaction. Hess’ law is based on the addition of reactions. Identify the name of the.
Solved A chemist measures the enthalpy change ΔH during the
Hess’ law is based on the addition of reactions. Identify the name of the enthalpy change for each reaction. To fairly compare the changes in enthalpy between reactions, all reactions should be carried out. Write the overall reaction, and identify the name of the enthalpy change for each reaction. Reaction enthalpy answer bank m(s) m(g) step 1:
Reaction Enthalpy Thermodynamics Physical Chemistry Chemistry
Write the overall reaction, and identify the name of the enthalpy change for each reaction. Reaction enthalpy answer bank m(s) m(g) step 1: Hess’ law is based on the addition of reactions. Identify the name of the enthalpy change for each reaction. To fairly compare the changes in enthalpy between reactions, all reactions should be carried out.
Enthalpy Profile Diagram Endothermic Reaction
Identify the name of the enthalpy change for each reaction. To fairly compare the changes in enthalpy between reactions, all reactions should be carried out. Reaction enthalpy answer bank m(s) m(g) step 1: By knowing the reaction enthalpy for constituent reactions, the enthalpy of a reaction that can be. Write the overall reaction, and identify the name of the enthalpy.
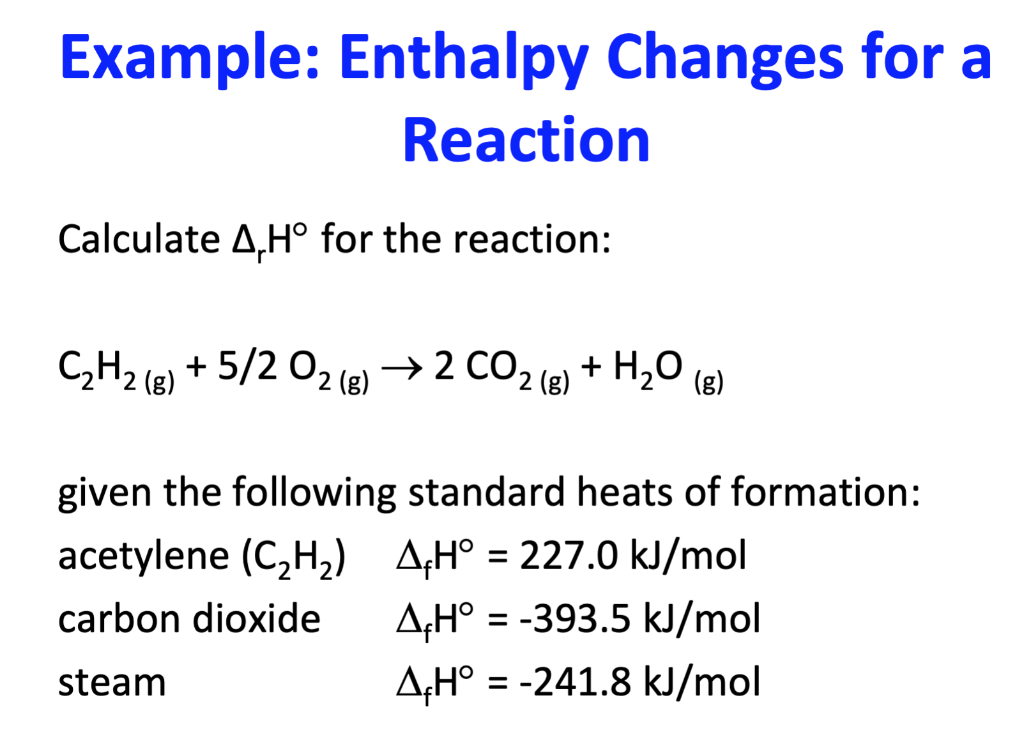
Solved Example Enthalpy Changes for a Reaction Calculate 4,
Reaction enthalpy answer bank m(s) m(g) step 1: Hess’ law is based on the addition of reactions. To fairly compare the changes in enthalpy between reactions, all reactions should be carried out. By knowing the reaction enthalpy for constituent reactions, the enthalpy of a reaction that can be. Identify the name of the enthalpy change for each reaction.
Standard Enthalpy Change Equation
By knowing the reaction enthalpy for constituent reactions, the enthalpy of a reaction that can be. To fairly compare the changes in enthalpy between reactions, all reactions should be carried out. Write the overall reaction, and identify the name of the enthalpy change for each reaction. Identify the name of the enthalpy change for each reaction. Reaction enthalpy answer bank.
Reaction Enthalpy Answer Bank M(S) M(G) Step 1:
Hess’ law is based on the addition of reactions. Identify the name of the enthalpy change for each reaction. Write the overall reaction, and identify the name of the enthalpy change for each reaction. To fairly compare the changes in enthalpy between reactions, all reactions should be carried out.