Ipledge Rems Fact Sheet
Ipledge Rems Fact Sheet - The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. What is the ipledge program? What is a risk evaluation and mitigation strategy (rems)? To avoid serious risks to unborn babies, the food and drug administration (fda) has required a special program called a risk evaluation and. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. What is the ipledge rems? To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. A rems is a risk management program that uses risk. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special.
What is the ipledge program? To avoid serious risks to unborn babies, the food and drug administration (fda) has required a special program called a risk evaluation and. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. A rems is a risk management program that uses risk. What is the ipledge rems? The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is a risk evaluation and mitigation strategy (rems)?
To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. What is a risk evaluation and mitigation strategy (rems)? To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge program? A rems is a risk management program that uses risk. To avoid serious risks to unborn babies, the food and drug administration (fda) has required a special program called a risk evaluation and. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. What is the ipledge rems?
Fillable Online iPledge REMS Frequently Asked Questions (FAQ) Fax
What is a risk evaluation and mitigation strategy (rems)? What is the ipledge program? The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. To avoid serious risks to unborn babies, the food.
iPLEDGE® REMS Accutane®
What is a risk evaluation and mitigation strategy (rems)? What is the ipledge rems? The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. A rems is a risk management program.
Petition · Cancel iPLEDGE REMS Program so that isotretinoin patients
What is a risk evaluation and mitigation strategy (rems)? The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. A rems is a risk management program that uses risk. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is.
FDA Issues Updates to iPLEDGE® REMS Program PAAS National
A rems is a risk management program that uses risk. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge rems? The ipledge rems is a safety program to manage.
FDA Announces Modifications to iPLEDGE REMS Program MPR
A rems is a risk management program that uses risk. What is the ipledge program? The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge rems?
Fillable Online iPledge REMS Frequently Asked Questions (FAQ
The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. What is the ipledge program? What is a risk evaluation and mitigation strategy (rems)? To avoid serious risks to unborn.
Accutane Ipledge Registration
The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure. The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. What is a risk evaluation and mitigation strategy (rems)? To avoid serious risks to unborn babies (fetus), the food and.
IPledge Isotretinoin REMS Prompts FDA Flexibility & Plea to Manufacturers
What is the ipledge program? To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. A rems is a risk management program that uses risk. The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. What is a risk evaluation and.
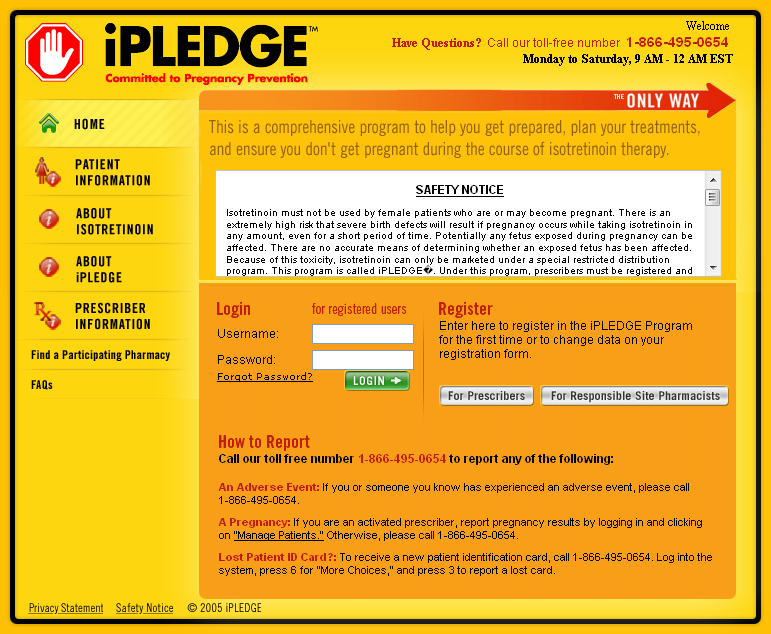
iPledge Login First Time at ️ [2023]
To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge program? To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is a risk evaluation and mitigation strategy (rems)? The ipledge rems is a safety program to manage the.
iPLEDGE allows athome pregnancy tests during pandemic The Hospitalist
What is a risk evaluation and mitigation strategy (rems)? What is the ipledge program? To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. The ipledge rems is a safety program to manage the.
What Is A Risk Evaluation And Mitigation Strategy (Rems)?
The goals of the ipledge rems are to prevent fetal exposure to isotretinoin and to inform prescribers, pharmacists, and patients about. To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge program? A rems is a risk management program that uses risk.
To Avoid Serious Risks To Unborn Babies, The Food And Drug Administration (Fda) Has Required A Special Program Called A Risk Evaluation And.
To avoid serious risks to unborn babies (fetus), the food and drug administration (fda) has required a special. What is the ipledge rems? The ipledge rems is a safety program to manage the risk of isotretinoin’s teratogenicity and to minimize fetal exposure.







![iPledge Login First Time at ️ [2023]](https://mytakesurvery.b-cdn.net/wp-content/uploads/2022/10/ipledge-login.jpg)
