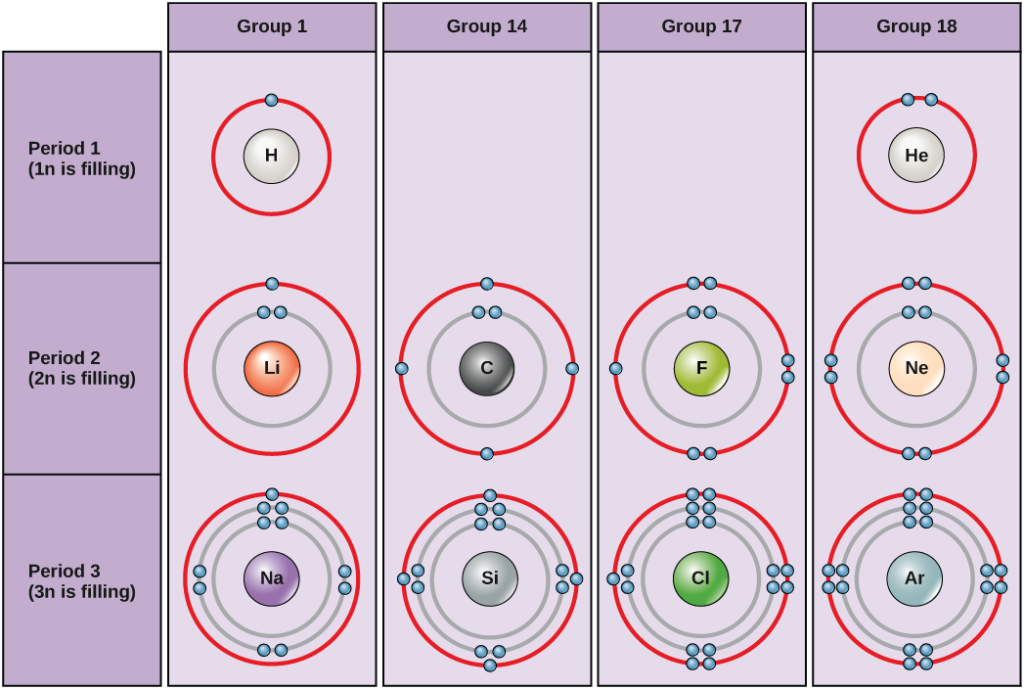
What Is A Stable Electron Configuration
What Is A Stable Electron Configuration - Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. Electron configuration can predict the stability of an atom. What is a stable electron configuration? The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. When an atom’s all orbitals are filled, it becomes.
Electron configuration can predict the stability of an atom. When an atom’s all orbitals are filled, it becomes. Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. What is a stable electron configuration? The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons.
Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. When an atom’s all orbitals are filled, it becomes. Electron configuration can predict the stability of an atom. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. What is a stable electron configuration?
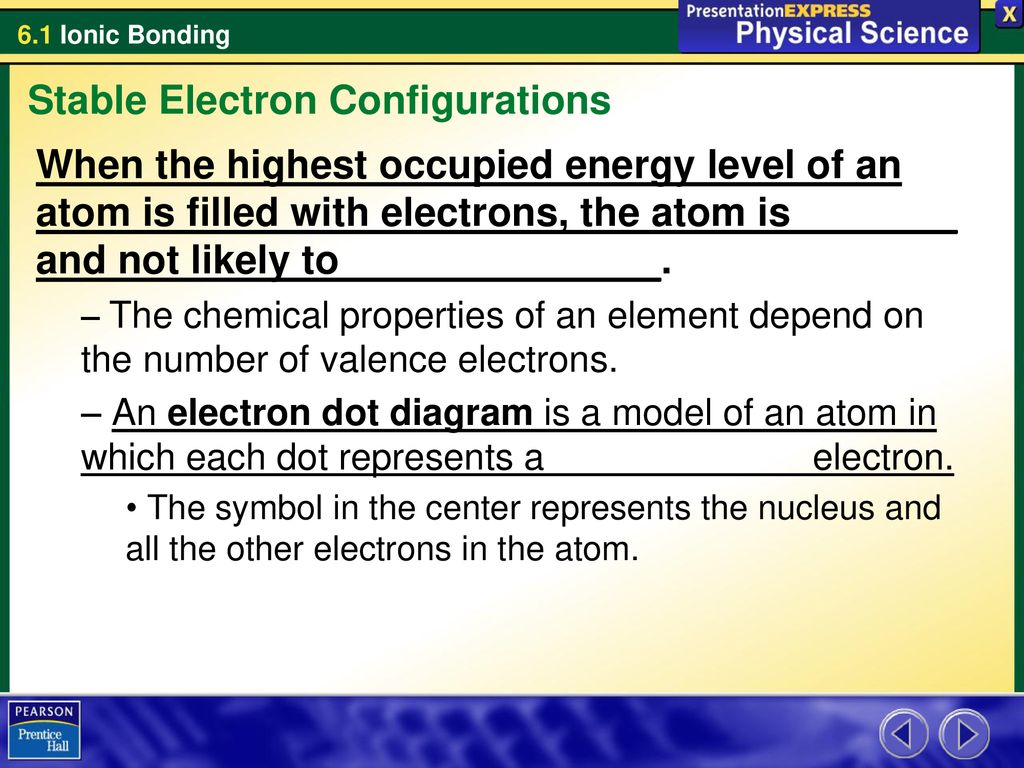
Stable Electron Configurations ppt download
Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. When an atom’s all orbitals are filled, it becomes. Electron configuration can predict the stability of an atom. What is a stable electron configuration? The octet rule states that atoms tend to gain, lose, or share electrons in.
Does the Lewis structure below show a stable electron configuration
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. Electron configuration can predict the stability of an atom. What is a stable electron configuration? When an atom’s all orbitals are filled, it becomes. Every system tends to acquire a state of stability or a state.
Electronic Configurations Intro Chemistry LibreTexts
What is a stable electron configuration? Electron configuration can predict the stability of an atom. Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. When an atom’s all orbitals are filled, it becomes. The octet rule states that atoms tend to gain, lose, or share electrons in.
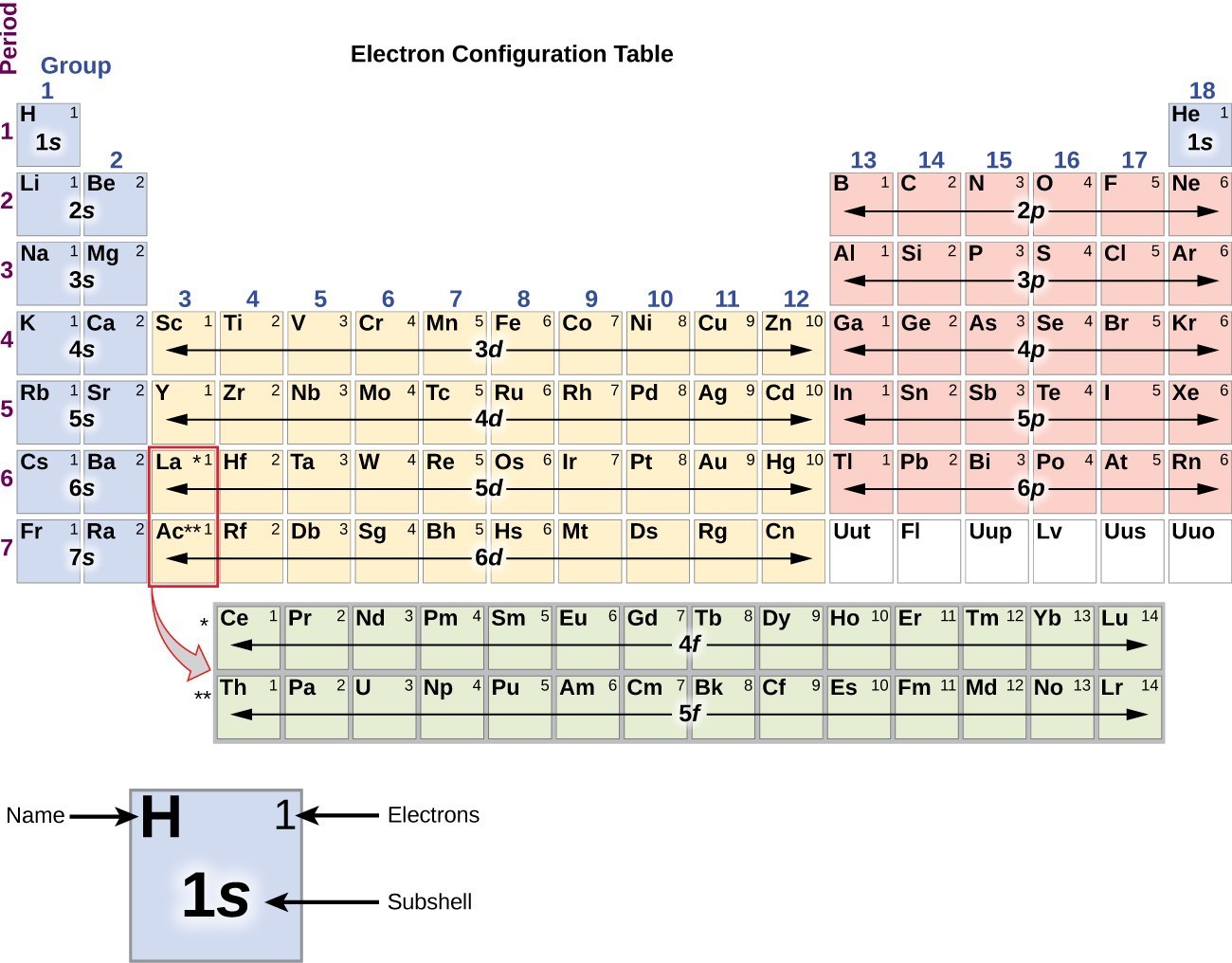
Electronic Structure of Atoms (Electron Configurations) Chemistry
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. Electron configuration can predict the stability of an atom. When an atom’s all orbitals are filled, it becomes. Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements.
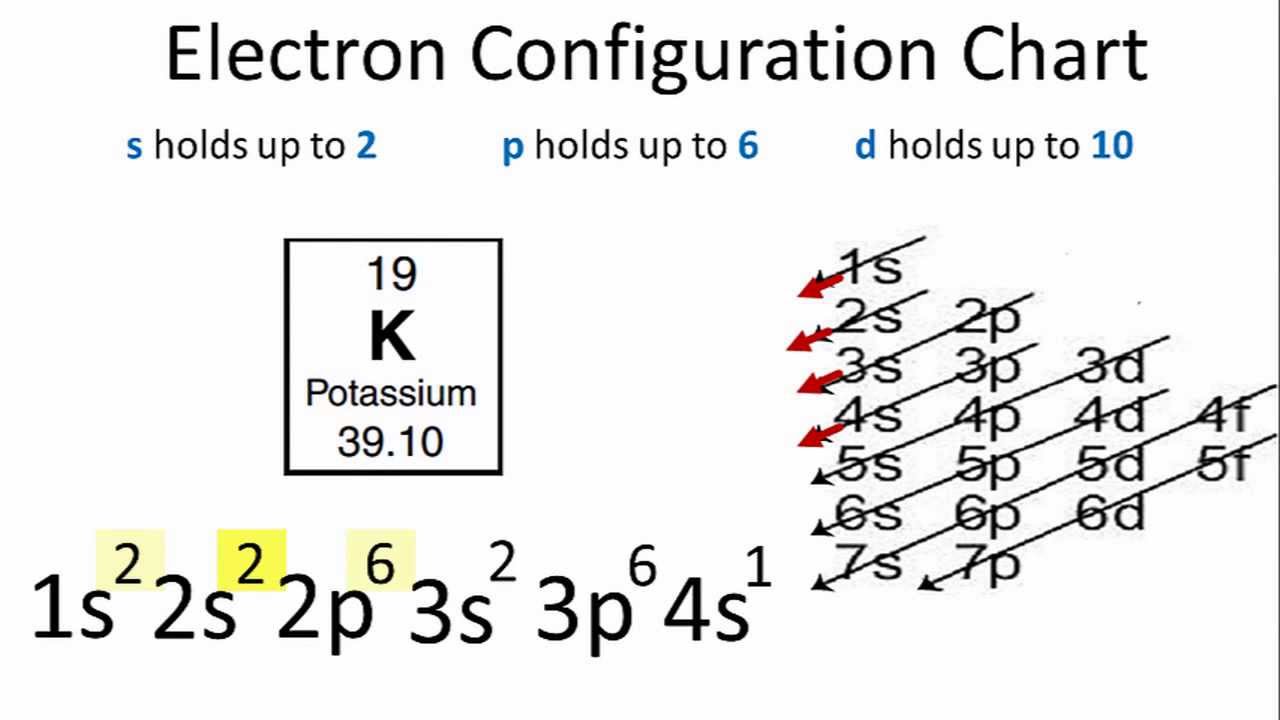
😍 Electron configuration examples. Abbreviated Electron configurations
What is a stable electron configuration? The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. When an atom’s all orbitals are filled, it.
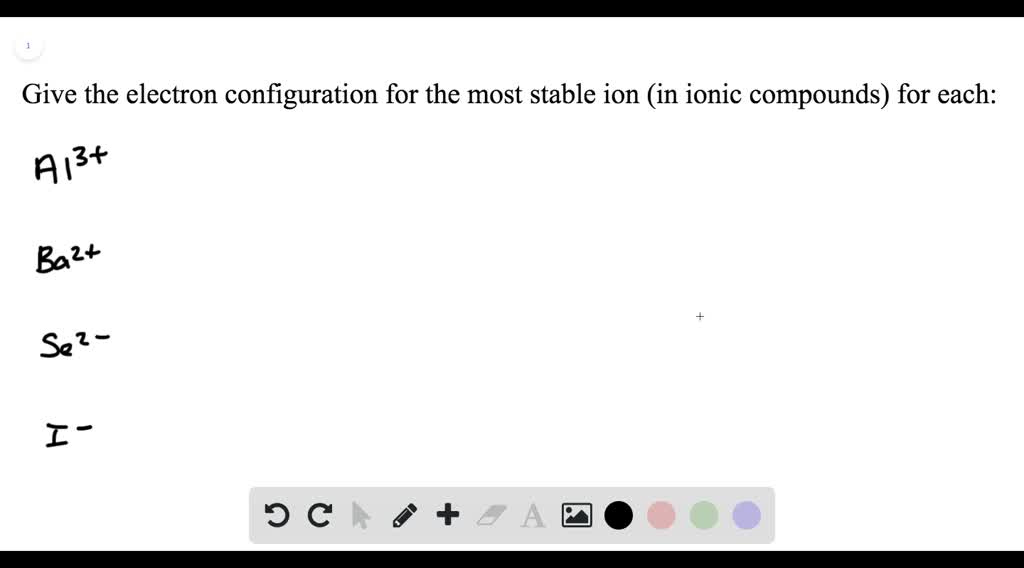
SOLVED Write electron configurations for the most stable ion formed by
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. Electron configuration can predict the stability of an atom. When an atom’s all orbitals.
Electrons Biology for Majors I
Electron configuration can predict the stability of an atom. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. When an atom’s all orbitals.
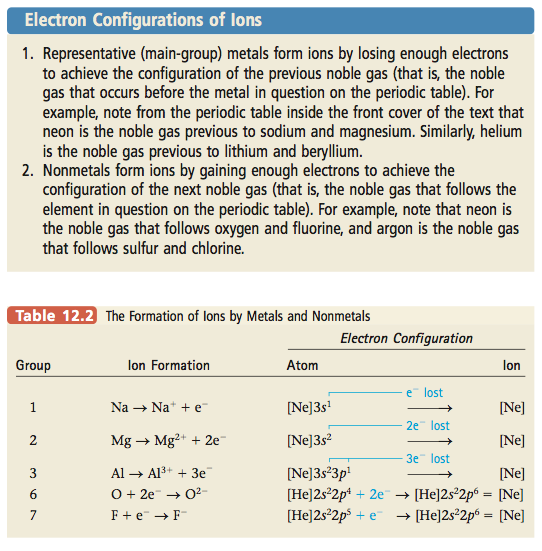
12.4 Stable Electron Configurations and Charges on Ions
Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. Electron configuration can predict the stability of an atom. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. When an atom’s all orbitals.
Periodic Table Stable Electron Configuration, HD Png Download vhv
Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. Electron configuration can predict the stability of an atom. When an atom’s all orbitals are filled, it becomes. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration.
Stable Electron Configurations YouTube
What is a stable electron configuration? Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. Electron configuration can predict the stability of an atom. When an atom’s all orbitals are filled, it becomes. The octet rule states that atoms tend to gain, lose, or share electrons in.
What Is A Stable Electron Configuration?
Every system tends to acquire a state of stability or a state of minimum energy, so chemical elements take part in chemical. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. When an atom’s all orbitals are filled, it becomes. Electron configuration can predict the stability of an atom.