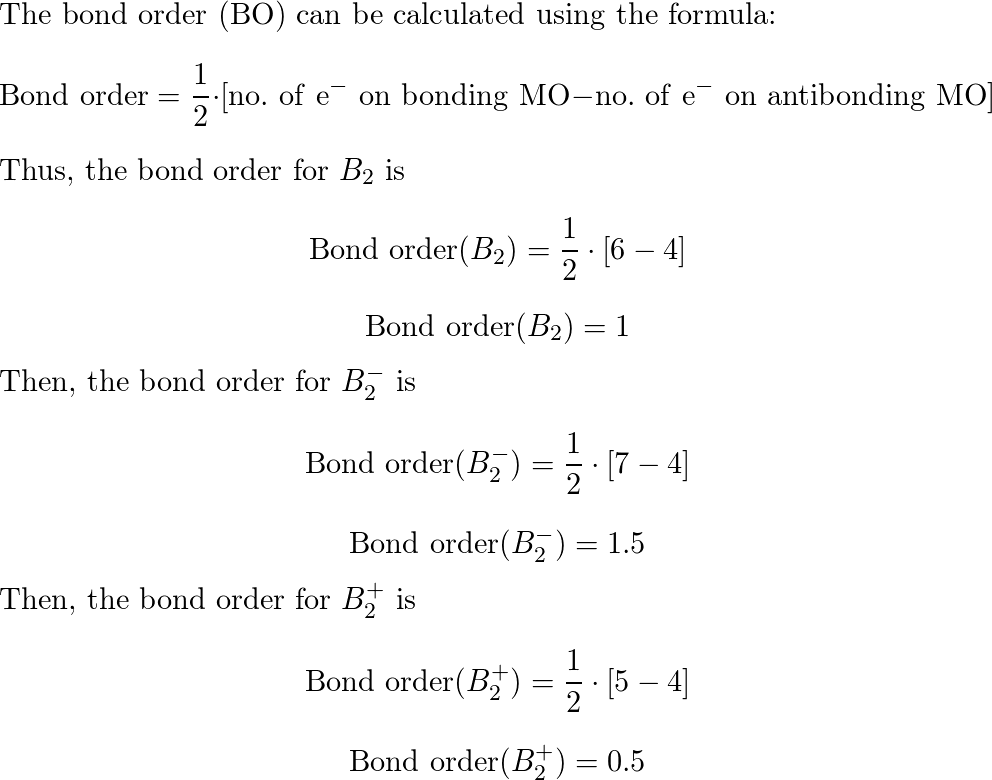What Is The Bond Order Of B2
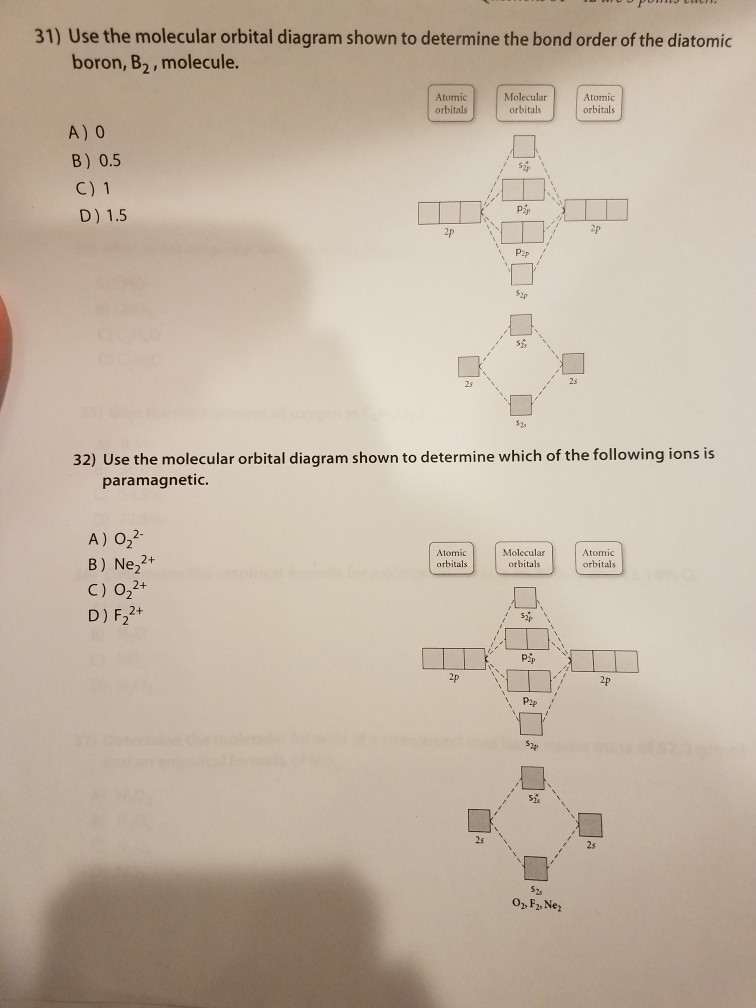
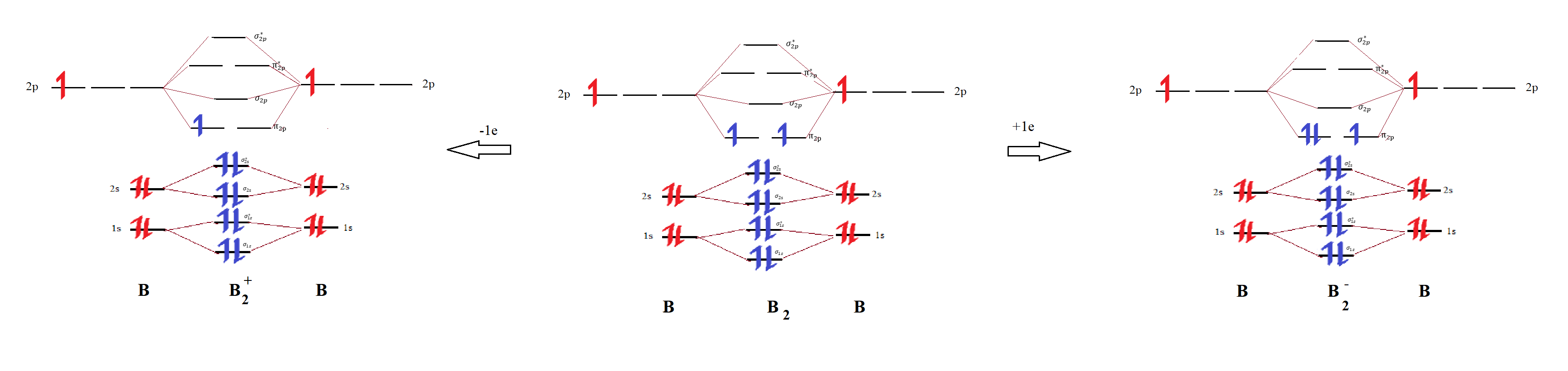
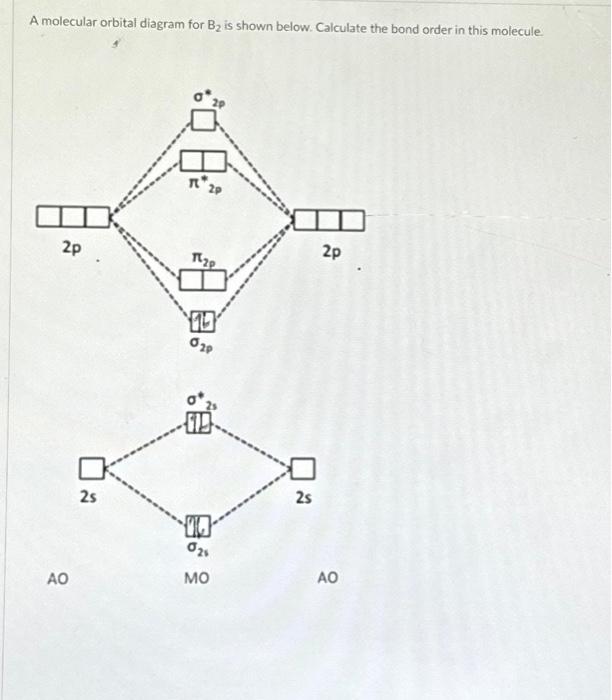
What Is The Bond Order Of B2 - Bonding orbitals are marked with sigma or pi and antibonding orbitals with sigma* or pi*. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. The b 2 molecule is known to be formed by the combination of two boron atoms, where they are linked by a covalent bond. This means that the electrons are effectively shared. The bond order of b 2 molecule: A bond order of 2 indicates a stable and strong bond between the boron atoms in the b2 molecule.
A bond order of 2 indicates a stable and strong bond between the boron atoms in the b2 molecule. The b 2 molecule is known to be formed by the combination of two boron atoms, where they are linked by a covalent bond. Bonding orbitals are marked with sigma or pi and antibonding orbitals with sigma* or pi*. The bond order of b 2 molecule: This means that the electrons are effectively shared. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding.
The bond order of b 2 molecule: Bonding orbitals are marked with sigma or pi and antibonding orbitals with sigma* or pi*. This means that the electrons are effectively shared. A bond order of 2 indicates a stable and strong bond between the boron atoms in the b2 molecule. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. The b 2 molecule is known to be formed by the combination of two boron atoms, where they are linked by a covalent bond.
Solved 31) Use the molecular orbital diagram shown to
By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. A bond order of 2 indicates a stable and strong bond between the boron atoms in the b2 molecule. Bonding orbitals are marked with sigma or pi and antibonding orbitals with sigma* or pi*. The b.
붕소 루이스 점자점식 지식iN
The bond order of b 2 molecule: A bond order of 2 indicates a stable and strong bond between the boron atoms in the b2 molecule. This means that the electrons are effectively shared. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. Bonding orbitals.
Molecular Orbital Diagram For B2
The bond order of b 2 molecule: This means that the electrons are effectively shared. A bond order of 2 indicates a stable and strong bond between the boron atoms in the b2 molecule. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. Bonding orbitals.
16. Explain Bond order of B2.
By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. The bond order of b 2 molecule: Bonding orbitals are marked with sigma or pi and antibonding orbitals with sigma* or pi*. The b 2 molecule is known to be formed by the combination of two.
F2 Molecular Orbital Diagram Bond Order
This means that the electrons are effectively shared. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. The bond order of b 2 molecule: The b 2 molecule is known to be formed by the combination of two boron atoms, where they are linked by.
Use MO diagrams to place B2+, B2, and B2 in order of decr Quizlet
The bond order of b 2 molecule: By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. The b 2 molecule is known to be formed by the combination of two boron atoms, where they are linked by a covalent bond. This means that the electrons.
Solved A molecular orbital diagram for B2 is shown below.
The bond order of b 2 molecule: The b 2 molecule is known to be formed by the combination of two boron atoms, where they are linked by a covalent bond. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. This means that the electrons.
BOND ORDER of B2 Molecule Bond order of BORON MOLECULE YouTube
Bonding orbitals are marked with sigma or pi and antibonding orbitals with sigma* or pi*. This means that the electrons are effectively shared. The b 2 molecule is known to be formed by the combination of two boron atoms, where they are linked by a covalent bond. By analyzing the mo diagram for b2, we can determine the bond order,.
define bond order and calculate the bond order in B2 Chemistry
This means that the electrons are effectively shared. The bond order of b 2 molecule: The b 2 molecule is known to be formed by the combination of two boron atoms, where they are linked by a covalent bond. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of.
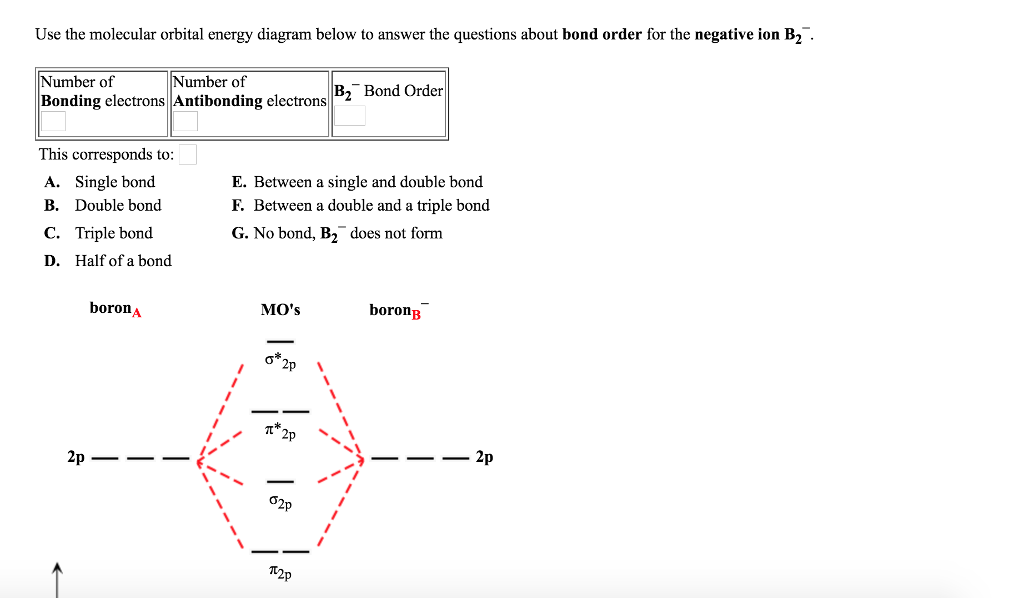
Solved Use the molecular orbital energy diagram below to
By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. Bonding orbitals are marked with sigma or pi and antibonding orbitals with sigma* or pi*. The b 2 molecule is known to be formed by the combination of two boron atoms, where they are linked by.
This Means That The Electrons Are Effectively Shared.
A bond order of 2 indicates a stable and strong bond between the boron atoms in the b2 molecule. By analyzing the mo diagram for b2, we can determine the bond order, which is the difference between the number of bonding and antibonding. Bonding orbitals are marked with sigma or pi and antibonding orbitals with sigma* or pi*. The bond order of b 2 molecule: