What Is The Charge On The Ion Formed By Aluminum
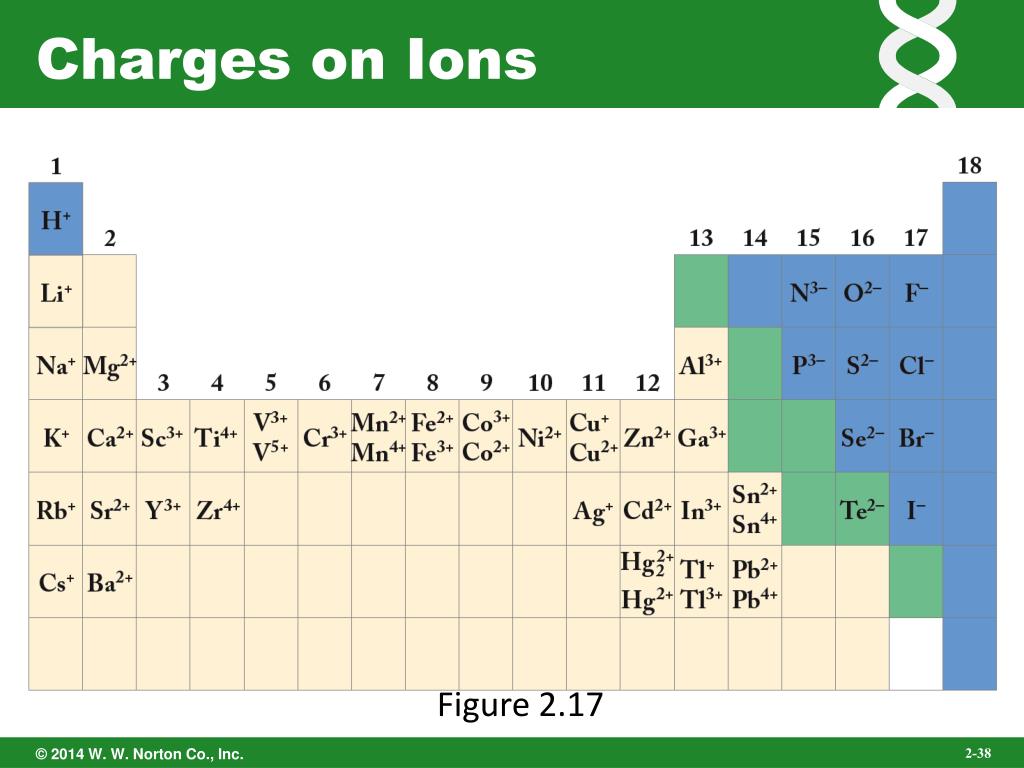
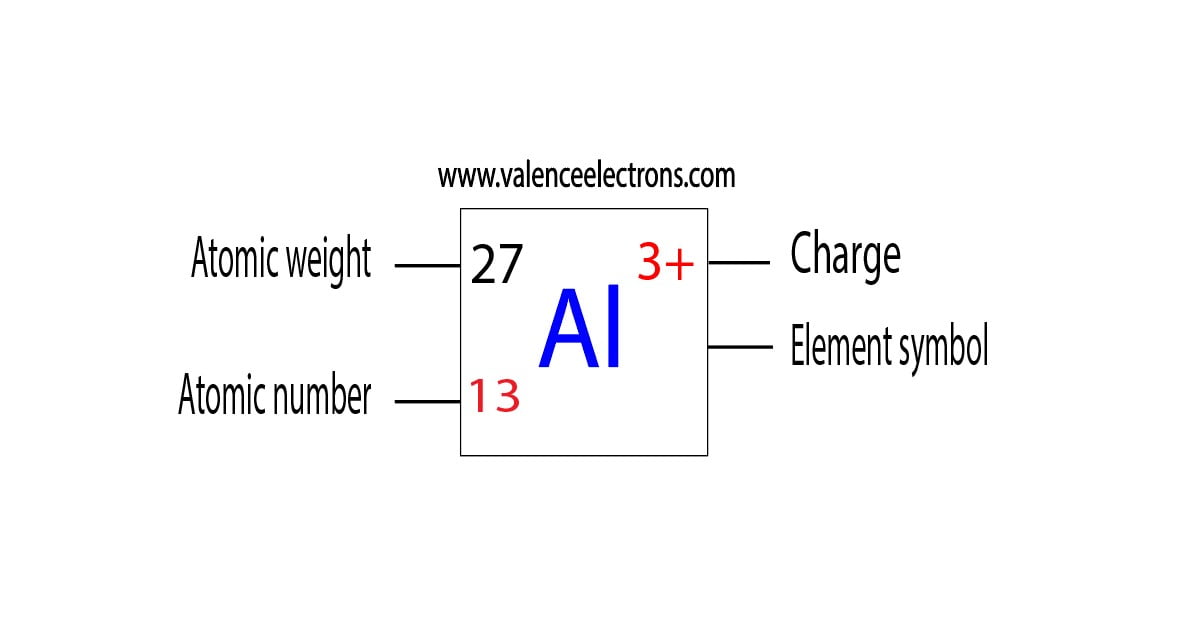
What Is The Charge On The Ion Formed By Aluminum - Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺). The charge of an aluminum ion is determined by its electron. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The charge of an aluminum ion is determined by its electron. Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺).
Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺). The charge of an aluminum ion is determined by its electron. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
What is the Charge on the Ion Formed by Aluminum? Aluminum Profile Blog
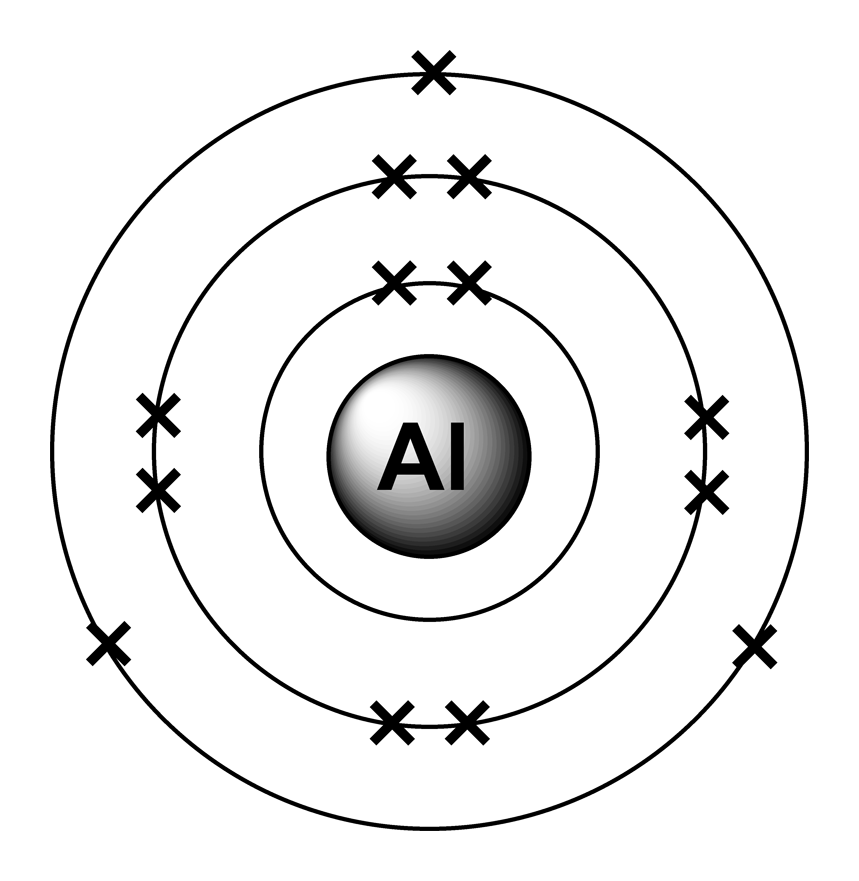
Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺). The charge of an aluminum ion is determined by its electron. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
How To Identify Charges Of Ions
The charge of an aluminum ion is determined by its electron. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺).
What is the Charge on the Ion Formed by Aluminum? Aluminum Profile Blog
When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺). The charge of an aluminum ion is determined by its electron.
What is the Charge on the Ion Formed by Aluminum? Aluminum Profile Blog
When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. The charge of an aluminum ion is determined by its electron. Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺).
What is the Charge on the Ion Formed by Aluminum? Aluminum Profile Blog
Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺). The charge of an aluminum ion is determined by its electron. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
Exploring Aluminum Ion Charge Properties, Characteristics and
Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺). The charge of an aluminum ion is determined by its electron. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
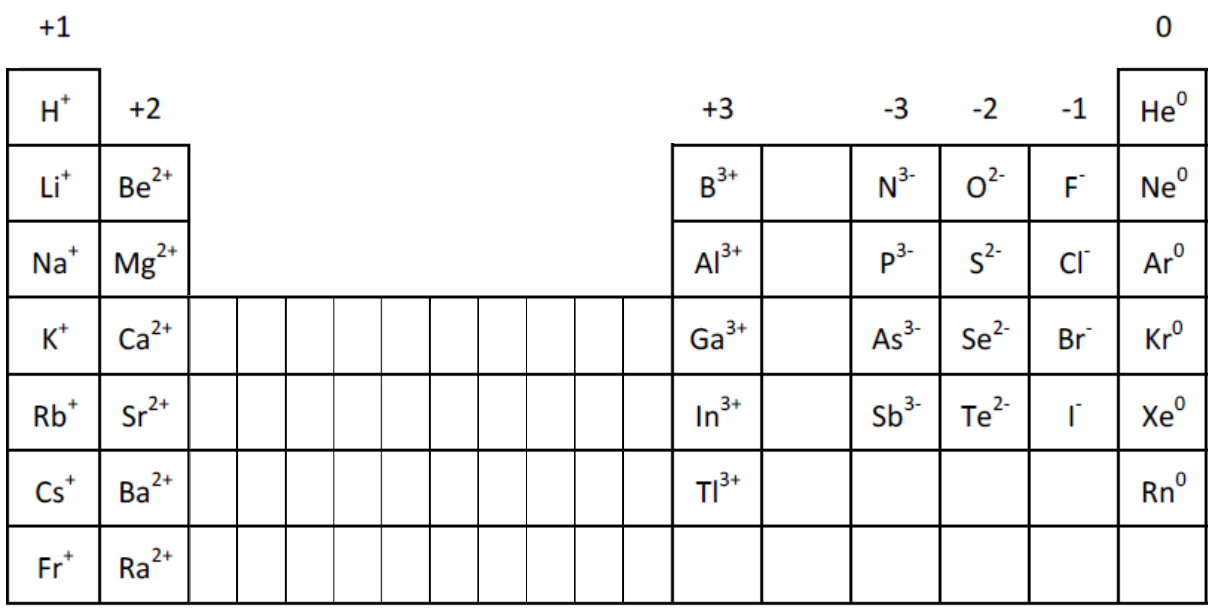
Ion Charge from Periodic Table NemoQuiz
The charge of an aluminum ion is determined by its electron. Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺). When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
Protons, Neutrons, Electrons for Aluminum (Al, Al3+)
The charge of an aluminum ion is determined by its electron. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺).
Periodic table with charged ions sciencetolf
When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺). The charge of an aluminum ion is determined by its electron.
When An Ionic Compound Is Formed From Magnesium And Oxygen, The Magnesium Ion Has A 2+ Charge, And The Oxygen Atom Has A 2− Charge.
The charge of an aluminum ion is determined by its electron. Aluminum ion loses 3 electrons, resulting in a charge of +3 (al³⁺).