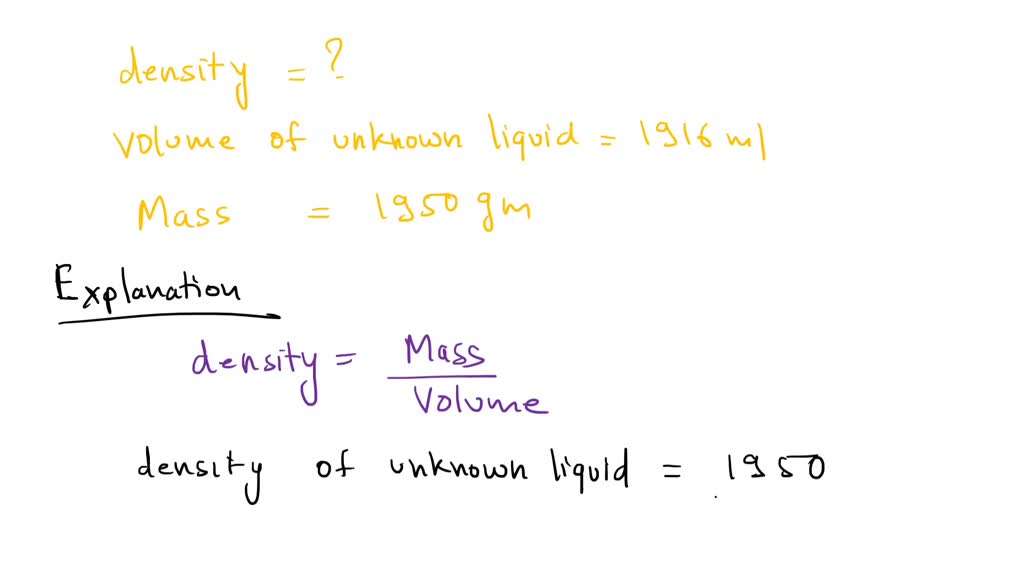What Is The Density Of Glycerol In G Cm3
What Is The Density Of Glycerol In G Cm3 - Density of aqueous solutions at 20°c, given as g/cm 3: The density of glycerin is 1.26 g/cm3. Glycerol is a syrupy liquid often used in cosmetics and soaps. 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10: Density of glycerol is equal to 1 261.3 kg/m³; If not provided, you may need to look it up in a reliable. What is the mass in g of 417 ml of ethylene glycol? Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. Typically, the density of glycerol is provided in units of grams per cubic centimeter (g/cm³).
Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10: Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. What is the mass in g of 417 ml of ethylene glycol? Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with. Density of aqueous solutions at 20°c, given as g/cm 3: If not provided, you may need to look it up in a reliable. Glycerol is a syrupy liquid often used in cosmetics and soaps. What is the volume in l of 4.1. Typically, the density of glycerol is provided in units of grams per cubic centimeter (g/cm³). 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e.
The density of glycerin is 1.26 g/cm3. Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10: What is the mass in g of 417 ml of ethylene glycol? Density of glycerol is equal to 1 261.3 kg/m³; Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with. Glycerol is a syrupy liquid often used in cosmetics and soaps. Density of aqueous solutions at 20°c, given as g/cm 3: Typically, the density of glycerol is provided in units of grams per cubic centimeter (g/cm³). What is the volume in l of 4.1. 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e.
SOLVED Next the chemist measures the volume of the unknown liquid as 0
Glycerol is a syrupy liquid often used in cosmetics and soaps. What is the volume in l of 4.1. What is the mass in g of 417 ml of ethylene glycol? If not provided, you may need to look it up in a reliable. Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance.
Solved A 20.0 mL sample of glycerol has a mass of 25.2
Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with. What is the volume in l of 4.1. Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. Typically, the density of glycerol is provided in units of grams per cubic centimeter (g/cm³). Density of aqueous solutions at 20°c, given as g/cm 3:
Glycerol monostearate Henan Eshine Chemicals Co.,Ltd
What is the volume in l of 4.1. Glycerol is a syrupy liquid often used in cosmetics and soaps. Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. Typically, the density of glycerol is provided in units of grams per cubic centimeter (g/cm³). What is the mass in g of 417 ml of ethylene glycol?
[Solved] A chemistry student needs 30.0 g of glycerol for an experiment
What is the volume in l of 4.1. If not provided, you may need to look it up in a reliable. Density of aqueous solutions at 20°c, given as g/cm 3: Density of glycerol is equal to 1 261.3 kg/m³; Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with.
Density of Glycerol {🪨 2022 update}
Glycerol is a syrupy liquid often used in cosmetics and soaps. The density of glycerin is 1.26 g/cm3. If not provided, you may need to look it up in a reliable. Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with. Ethylene glycol (antifreeze) has a density of 1.11 g>cm3.
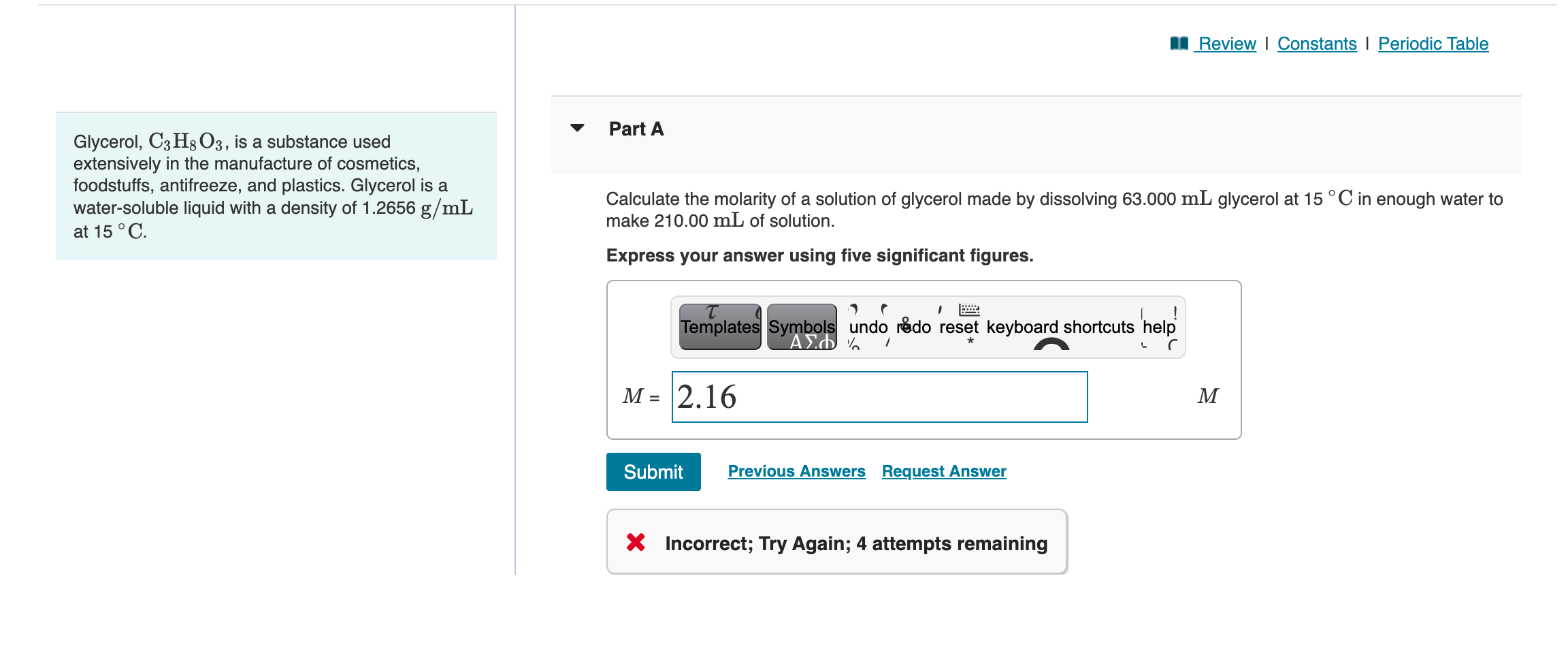
Solved Glycerol, C3H8O3, is a substance used Part A
The density of glycerin is 1.26 g/cm3. Glycerol is a syrupy liquid often used in cosmetics and soaps. Density of aqueous solutions at 20°c, given as g/cm 3: Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10:
SOLVED What is the density of 2 glycerol, given that the density of
The density of glycerin is 1.26 g/cm3. Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10: Density of glycerol is equal to 1 261.3 kg/m³; What is the mass in g of 417 ml of ethylene glycol? If not provided, you may need to look it up in a reliable.
Glycerol density gradient centrifugation to determine PKM2 oligomers
If not provided, you may need to look it up in a reliable. What is the mass in g of 417 ml of ethylene glycol? 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with. The.
Glycerin’s relative density Glycerin Refinery Equipment
Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. Density of aqueous solutions at 20°c, given as g/cm 3: The density of glycerin is 1.26 g/cm3. What is the volume in l of 4.1. 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e.
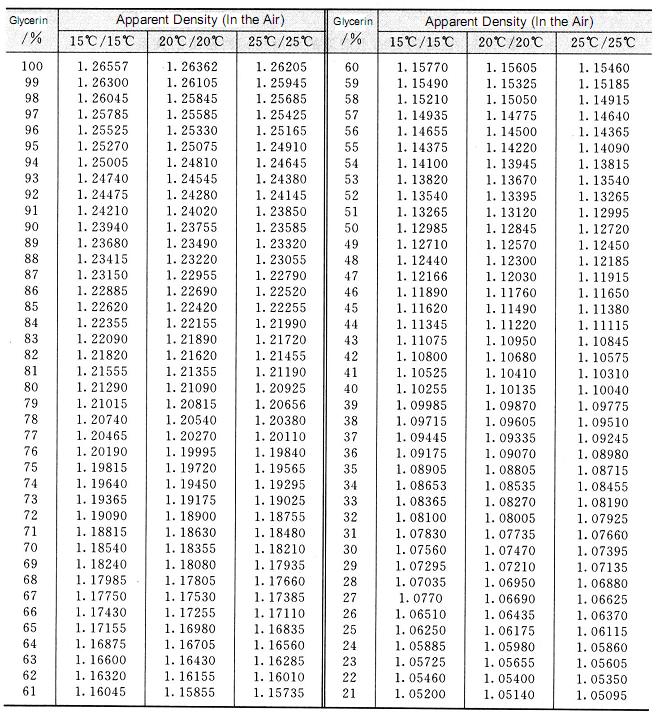
Densities (g cm 3 ) for glycerol + water mixtures at various
Density of aqueous solutions at 20°c, given as g/cm 3: 20 rows glycerol weighs 1.2613 gram per cubic centimeter or 1 261.3 kilogram per cubic meter, i.e. Glycerol is a syrupy liquid often used in cosmetics and soaps. What is the volume in l of 4.1. Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while.
Density Of Glycerol Is Equal To 1 261.3 Kg/M³;
Ethylene glycol (antifreeze) has a density of 1.11 g>cm3. If not provided, you may need to look it up in a reliable. The density of glycerin is 1.26 g/cm3. Any substance with a density greater than 1.26 g/cm3 will sink in glycerin, while a substance with.
What Is The Volume In L Of 4.1.
Density of aqueous solutions at 20°c, given as g/cm 3: Glycerol is a syrupy liquid often used in cosmetics and soaps. What is the mass in g of 417 ml of ethylene glycol? Glycerin 1.260 mercury 13.55 solutions sodium chloride in water grams solute/100 grams solution density (g/cm3) 10:
20 Rows Glycerol Weighs 1.2613 Gram Per Cubic Centimeter Or 1 261.3 Kilogram Per Cubic Meter, I.e.
Typically, the density of glycerol is provided in units of grams per cubic centimeter (g/cm³).