What Is The Molecular Geometry Of Ozone O3
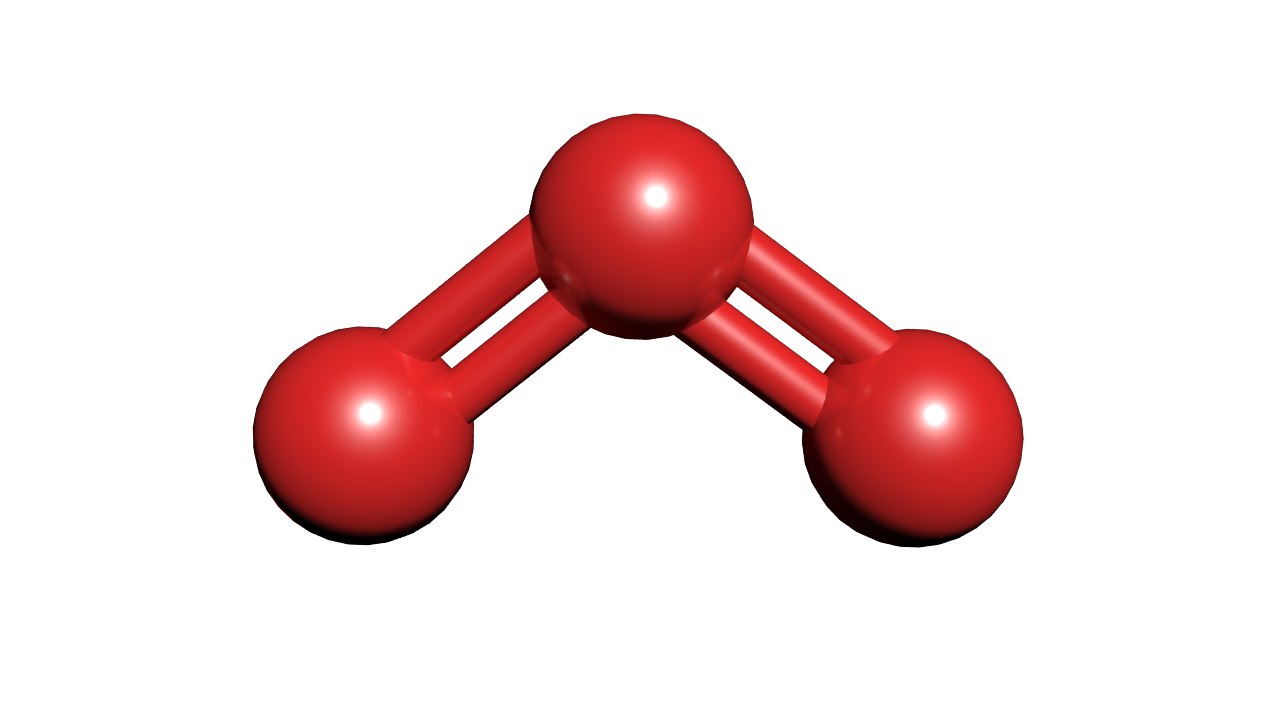
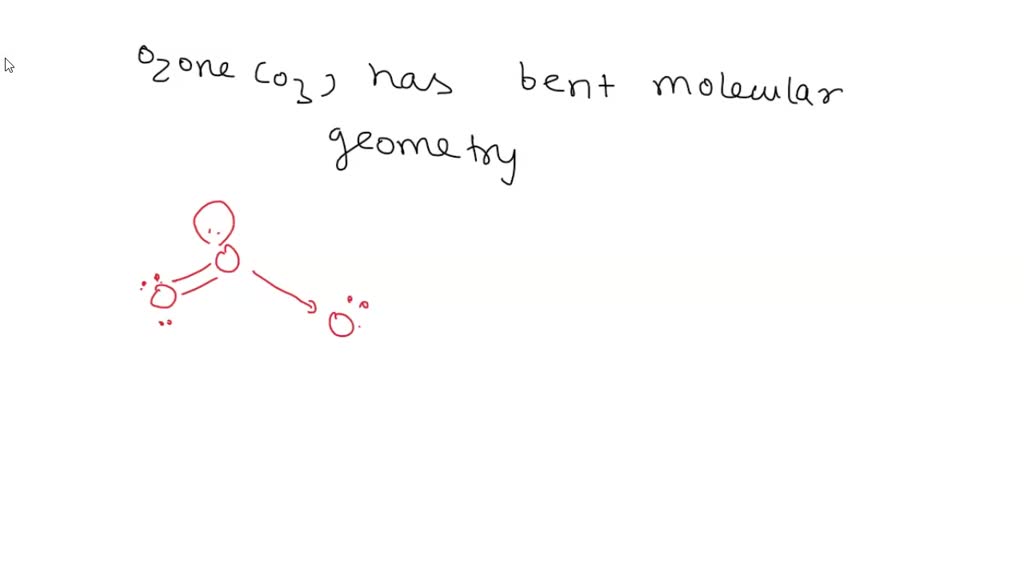
What Is The Molecular Geometry Of Ozone O3 - Repulsion causes the bond angle to. In the lewis structure of ozone, there are one double boond and one single bond. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen.
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Repulsion causes the bond angle to. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. In the lewis structure of ozone, there are one double boond and one single bond.
Repulsion causes the bond angle to. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. In the lewis structure of ozone, there are one double boond and one single bond. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance.
3D o3 molecule ozone model TurboSquid 1425810
Repulsion causes the bond angle to. In the lewis structure of ozone, there are one double boond and one single bond. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron.
SOLVED 1. Molecular shape of ozone (O3)? Molecular shape of ozone (O3
Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double boond and one single bond. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though.
O3 Molecular Geometry,Shape and Bond Angles(Ozone) Molecular geometry
In the lewis structure of ozone, there are one double boond and one single bond. Repulsion causes the bond angle to. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron.
O3 ozone molecule Royalty Free Vector Image VectorStock
Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double boond and one single bond. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. The ozone molecule is found to be.
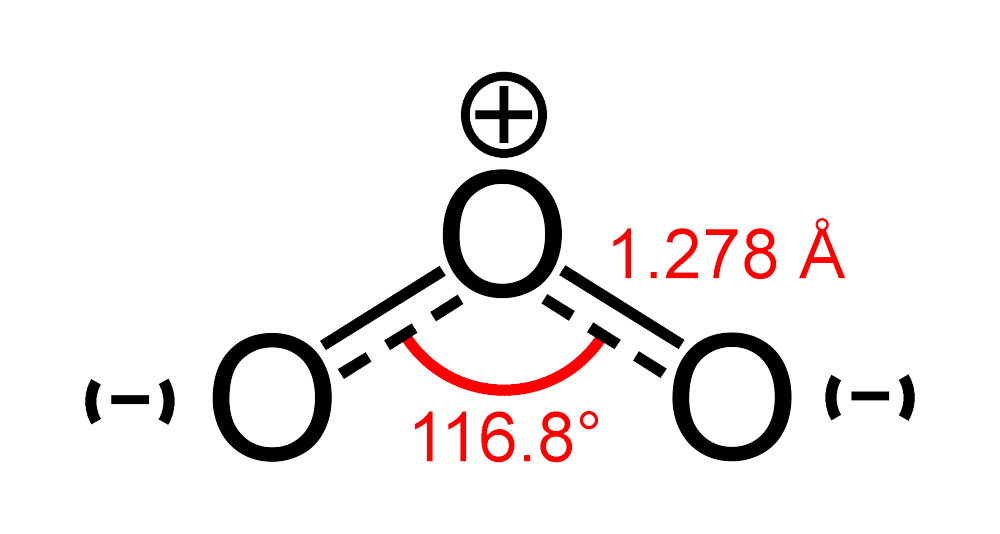
What is the \\angle OOO bond angle in ozone?
In the lewis structure of ozone, there are one double boond and one single bond. Repulsion causes the bond angle to. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. The.
3D O3 Molecule Ozone Model TurboSquid 1425810
In the lewis structure of ozone, there are one double boond and one single bond. Repulsion causes the bond angle to. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an example of a molecule whose electron.
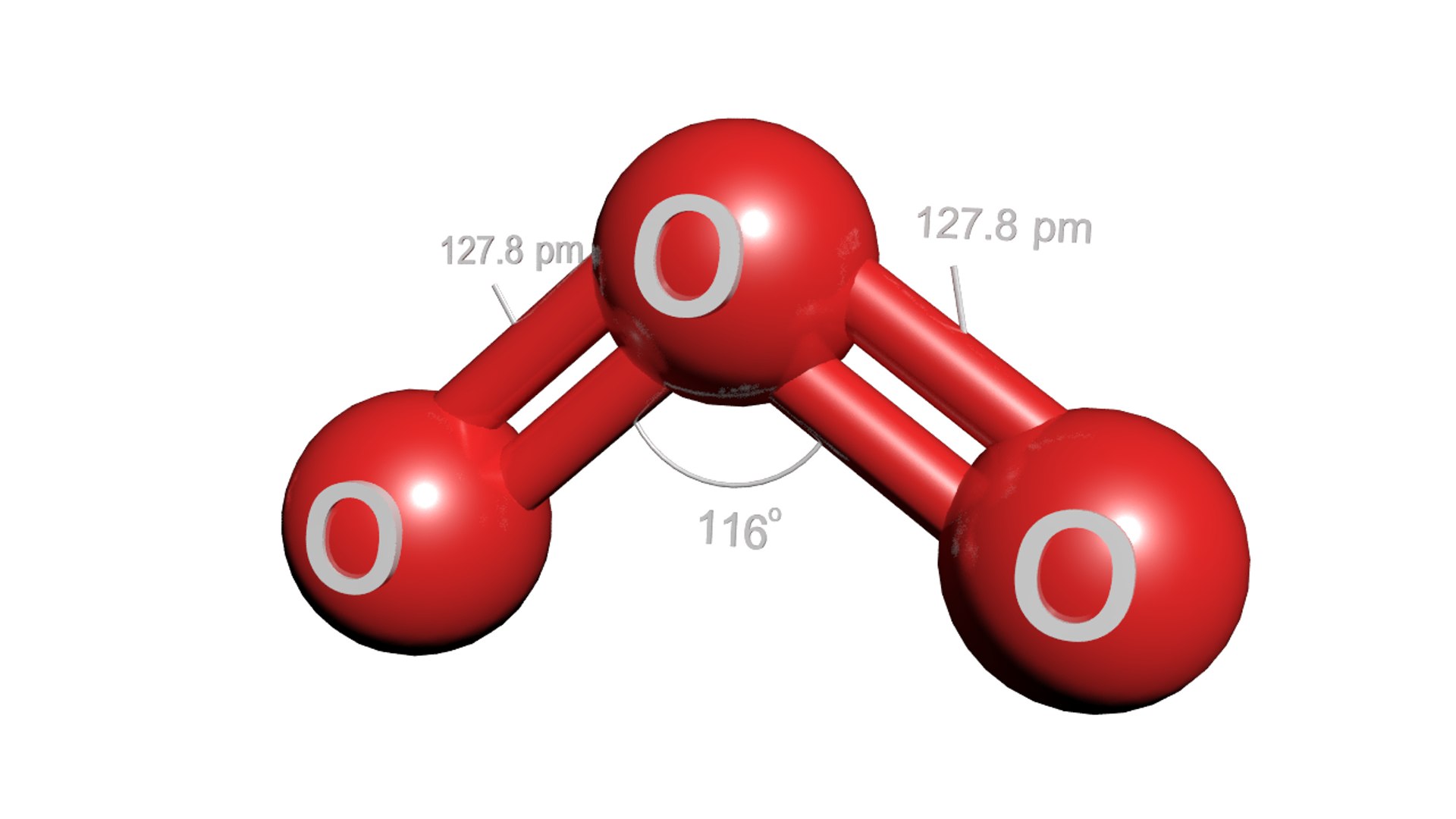
Which of the following is the correct molecular geometry for ozone. O3
In the lewis structure of ozone, there are one double boond and one single bond. Repulsion causes the bond angle to. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. The.
Is O3 Polar or Nonpolar? Techiescientist
Repulsion causes the bond angle to. In the lewis structure of ozone, there are one double boond and one single bond. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. The ozone molecule is found to be bent trigonal planar shape due to.
What is O3 lewis structure and how to calculate the formal charge on it
Repulsion causes the bond angle to. In the lewis structure of ozone, there are one double boond and one single bond. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. The.
Ozone O3 Molecule Stock Photo Alamy
Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Repulsion causes the bond angle to. In the lewis structure of ozone, there are one double boond and one single bond. The.
Repulsion Causes The Bond Angle To.
In the lewis structure of ozone, there are one double boond and one single bond. The ozone molecule is found to be bent trigonal planar shape due to the presence of resonance. Ozone (o3) is an allotrope of oxygen and contains three oxygen atoms. Ozone (o3) is an example of a molecule whose electron domain geometry is trigonal planar, though the presence of a lone pair on the central oxygen.