Which Element Is Most Likely To Form Three Covalent Bonds
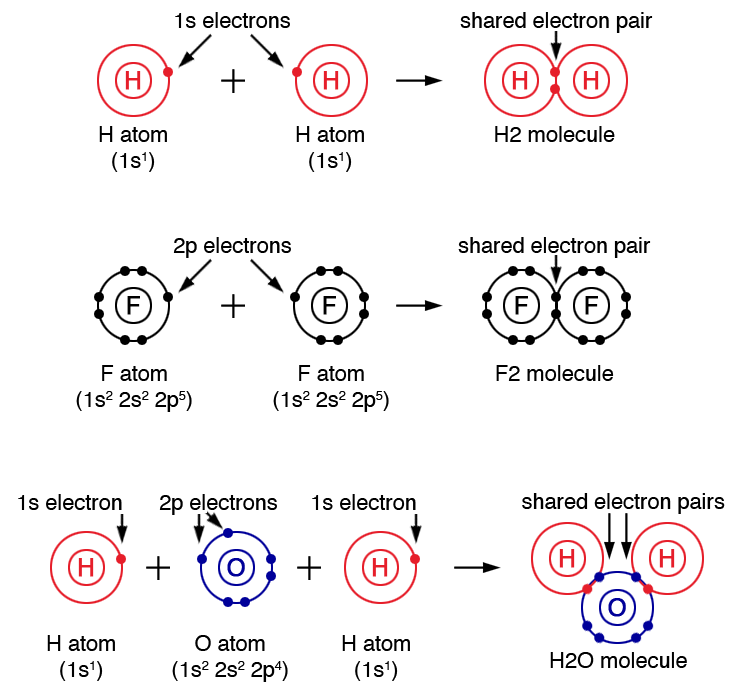
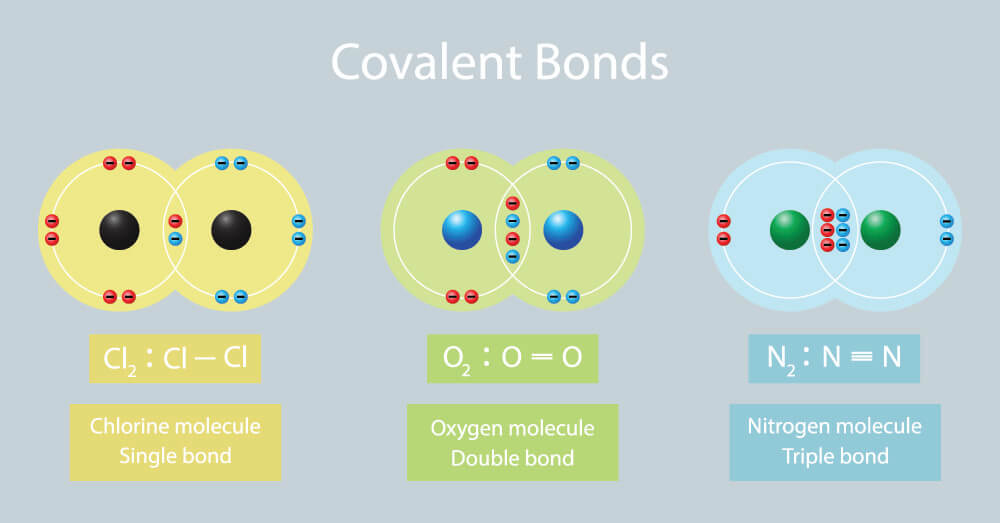
Which Element Is Most Likely To Form Three Covalent Bonds - The distance between two nuclei when repulsion and attraction are balanced. A) phosphorus b) nitrogen c) sulfur d) bromine For the dot structure shown the most likely elements are x =________ and y = ________. Which element is most likely to form three covalent bonds? To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). The most likely element to form three covalent bonds. Which element is most likely to make two covalent bonds in the formation of a molecular compound? After analyzing the properties of various elements, we can conclude that. The most common examples are the covalent compounds of beryllium and boron. Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the.
For example, beryllium can form two covalent bonds, resulting. Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the. Which element is most likely to make two covalent bonds in the formation of a molecular compound? A) phosphorus b) nitrogen c) sulfur d) bromine Which element is most likely to form three covalent bonds? The distance between two nuclei when repulsion and attraction are balanced. After analyzing the properties of various elements, we can conclude that. The most likely element to form three covalent bonds. For the dot structure shown the most likely elements are x =________ and y = ________. The most common examples are the covalent compounds of beryllium and boron.
For example, beryllium can form two covalent bonds, resulting. The most likely element to form three covalent bonds. For the dot structure shown the most likely elements are x =________ and y = ________. A) phosphorus b) nitrogen c) sulfur d) bromine Oxygen and other atoms in group 6a (16) obtain an octet. Which element is most likely to form three covalent bonds? The distance between two nuclei when repulsion and attraction are balanced. The most common examples are the covalent compounds of beryllium and boron. Which element is most likely to make two covalent bonds in the formation of a molecular compound? To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia).
Covalent Bonds Biology for Majors I
The distance between two nuclei when repulsion and attraction are balanced. Which element is most likely to form three covalent bonds? The most common examples are the covalent compounds of beryllium and boron. Which element is most likely to form three covalent. Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired.
Covalent Bonding (Biology) — Definition & Role Expii
After analyzing the properties of various elements, we can conclude that. For the dot structure shown the most likely elements are x =________ and y = ________. For example, beryllium can form two covalent bonds, resulting. Which element is most likely to form three covalent bonds? A) phosphorus b) nitrogen c) sulfur d) bromine
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
For the dot structure shown the most likely elements are x =________ and y = ________. Each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the. A) phosphorus b) nitrogen c) sulfur d) bromine Which element is most likely to make two covalent bonds in the formation.
Covalent bonds Learning Lab
Which element is most likely to form three covalent bonds? Which element is most likely to form three covalent. Which element is most likely to make two covalent bonds in the formation of a molecular compound? The most likely element to form three covalent bonds. For the dot structure shown the most likely elements are x =________ and y =.
Covalent Bonds Form When Valence Electrons
Which element is most likely to form three covalent. The most common examples are the covalent compounds of beryllium and boron. A) phosphorus b) nitrogen c) sulfur d) bromine After analyzing the properties of various elements, we can conclude that. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia).
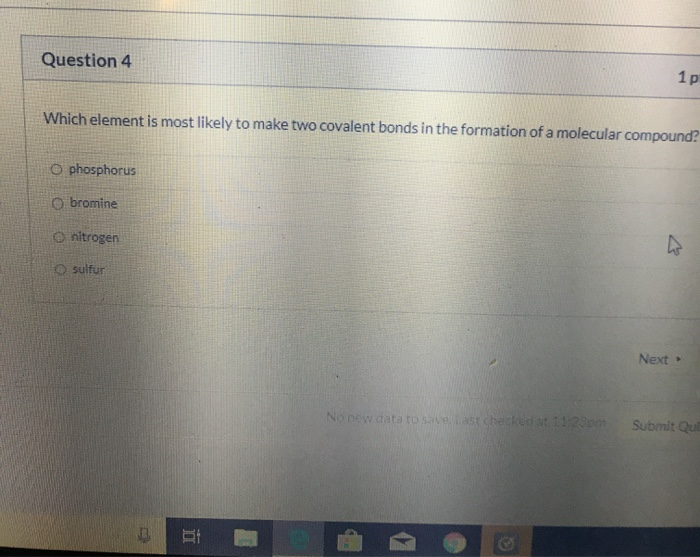
Solved which element is most likely to make two covalent
Which element is most likely to make two covalent bonds in the formation of a molecular compound? The distance between two nuclei when repulsion and attraction are balanced. Which element is most likely to form three covalent. A) phosphorus b) nitrogen c) sulfur d) bromine Oxygen and other atoms in group 6a (16) obtain an octet.
Question Video Identifying Pairs of Elements Likely to Bond Covalently
The distance between two nuclei when repulsion and attraction are balanced. To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Which element is most likely to form three covalent bonds? A) phosphorus b) nitrogen c) sulfur d) bromine Which element is most likely to form three covalent.
Which diagram shows how the covalent bonds most likely form in a
For example, beryllium can form two covalent bonds, resulting. The most common examples are the covalent compounds of beryllium and boron. A) phosphorus b) nitrogen c) sulfur d) bromine Which element is most likely to form three covalent. For the dot structure shown the most likely elements are x =________ and y = ________.
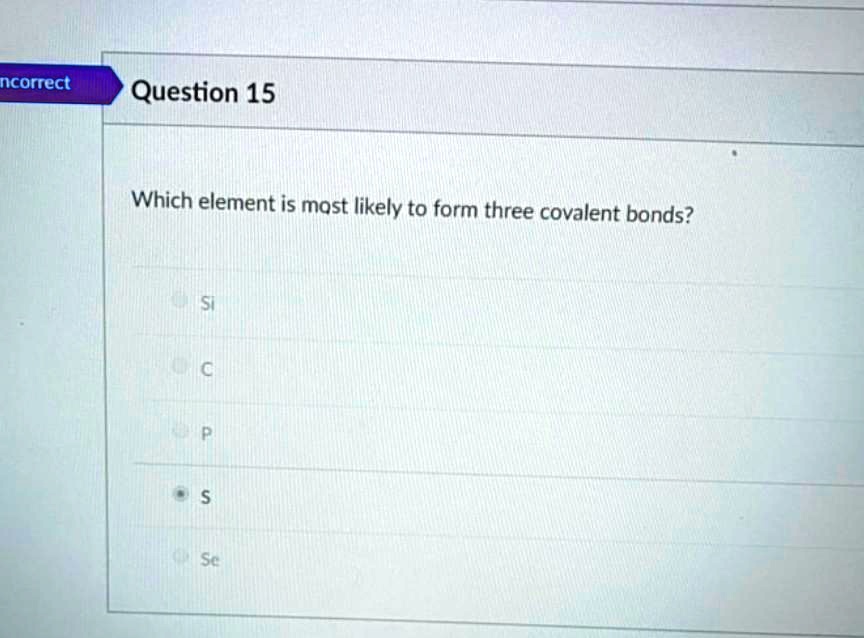
SOLVED Question 15 Which element is most likely to form three covalent
Oxygen and other atoms in group 6a (16) obtain an octet. For example, beryllium can form two covalent bonds, resulting. After analyzing the properties of various elements, we can conclude that. Which element is most likely to make two covalent bonds in the formation of a molecular compound? For the dot structure shown the most likely elements are x =________.
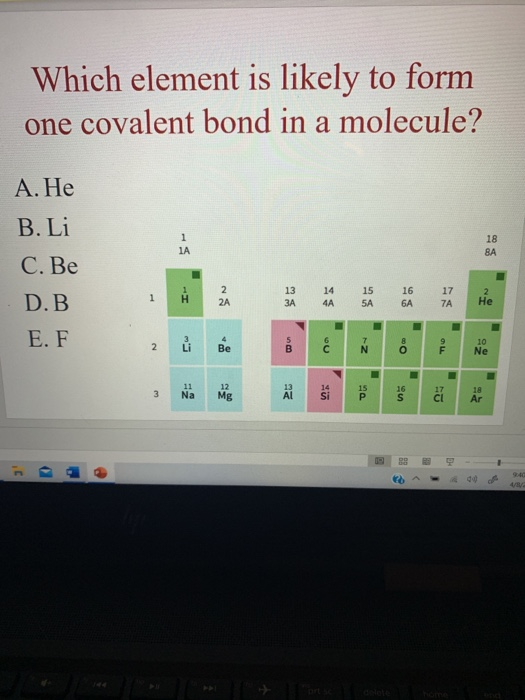
Solved Which element is likely to form one covalent bond in
Which element is most likely to form three covalent. The distance between two nuclei when repulsion and attraction are balanced. For example, beryllium can form two covalent bonds, resulting. Which element is most likely to make two covalent bonds in the formation of a molecular compound? The most likely element to form three covalent bonds.
For Example, Beryllium Can Form Two Covalent Bonds, Resulting.
Oxygen and other atoms in group 6a (16) obtain an octet. A) phosphorus b) nitrogen c) sulfur d) bromine To obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Which element is most likely to form three covalent bonds?
Each Of The 3 Chlorines Then Forms A Covalent Bond By Merging The Atomic Orbital Containing Its Unpaired Electron With One Of The.
The distance between two nuclei when repulsion and attraction are balanced. For the dot structure shown the most likely elements are x =________ and y = ________. After analyzing the properties of various elements, we can conclude that. The most likely element to form three covalent bonds.
Which Element Is Most Likely To Make Two Covalent Bonds In The Formation Of A Molecular Compound?
Which element is most likely to form three covalent. The most common examples are the covalent compounds of beryllium and boron.